Integrating patient training into product launches
The growing market for self-administered injectables calls for better patient-training resources
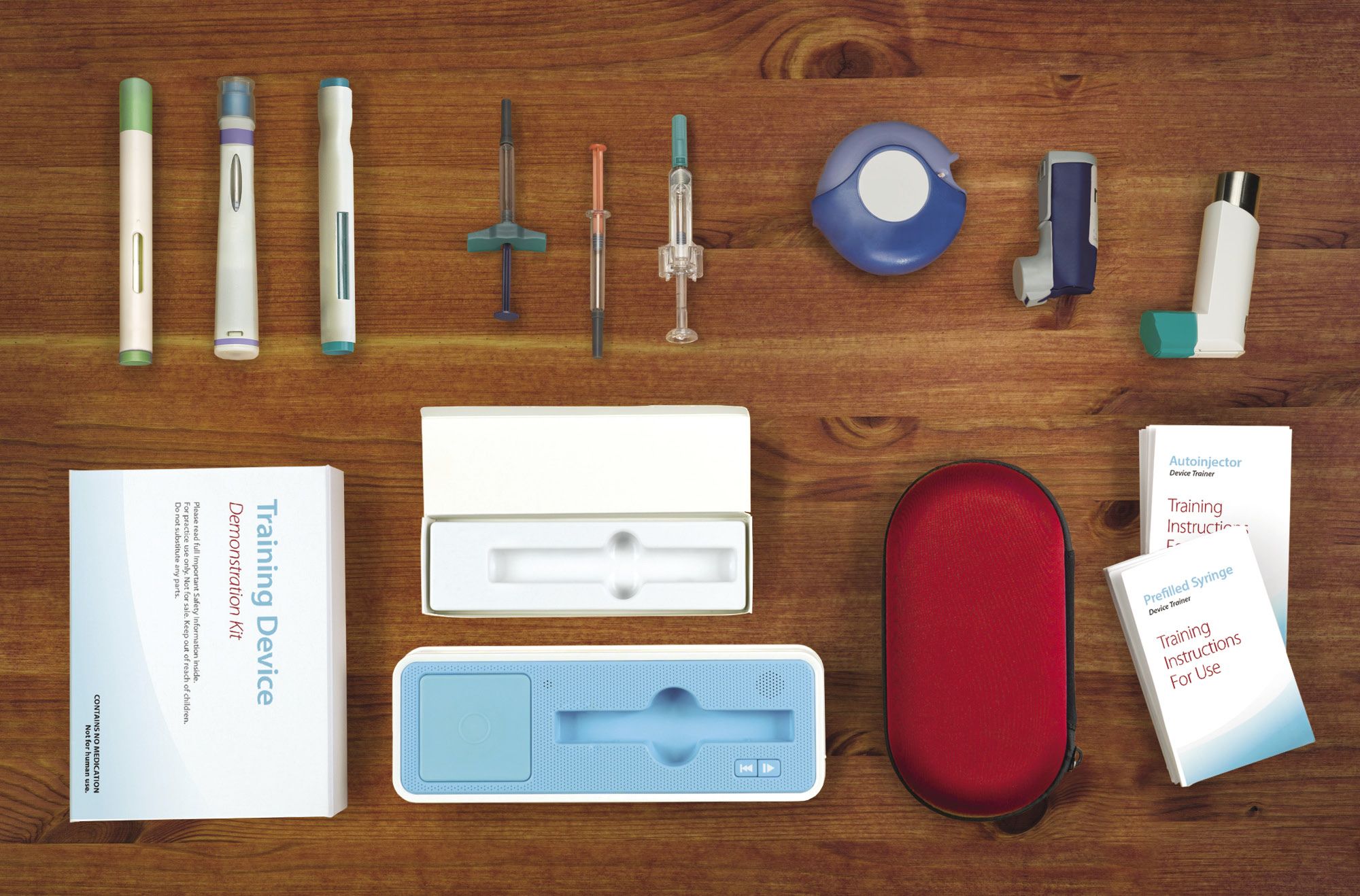
Fig. 1. Examples of the drug-delivery devices for which patient training is desirable. Source: Noble
Product launch is a critical moment in a brand’s ultimate success. Product launches for pharmaceuticals are complex and differ in many ways from other industries, with multiple key players such as research and development, formulation specialists, analysts, regulatory affairs, device engineers, commercial/brand teams, and many others intimately involved. This process takes on additional complexity with combination products—generally, drug-device combinations such as prefilled syringes, inhalation devices and the like.
To stand out from the crowd and to increase brand loyalty and adherence, an increasing number of pharmaceutical companies are offering more robust patient support programs that include educational and training tools in an effort to assist the growing number of patients required to self-administer injections. As part of the commercial launch strategy, patient training, education and support programs must be in place and ready to launch when the drug is launched.
The introduction of reusable drug-delivery device trainers has fostered an evolution of patient training platforms. Prior to these training technologies, the available training options outside a healthcare provider's office included Instructions for Use (IFU) and saline-filled prefilled syringes or autoinjectors, where users practice administration by injecting into an analog such as an orange. This process did not prove to be cost-effective and limited the amount of exposures for users to learn correct injection technique. In addition, it did not allow for vital learning experiences that help facilitate proper self-administration of an injection device, such as being familiar with a drug delivery device’s functionality, operational sequence and understanding of the IFU.
According to a study conducted by the University of Texas Medical Branch at Galveston (UTMB), 84% of patients failed to demonstrate correct use of an autoinjector and 92% failed to demonstrate correct use of an inhaler. [1] Another study found 45% of patients avoid injections due to anxiety. [2] Data suggests rates for consistent, daily use are also low. Additionally, a study conducted by Noble and researchers from Auburn University surveyed more than 700 patients who self-inject and found that more than half admitted they did not completely read the IFU prior to beginning treatment. [3] These findings suggest a lack of patient training could result in low adherence, errors, discontinuation of drug use and a decrease in brand loyalty.
To address the market need for patient training and education, reusable drug-delivery training devices and educational platforms have been developed. These drug-delivery device trainers are device-comparable solutions, which accurately replicate an actual drug delivery device’s form, functionality, operational sequence, haptic feedback, plunger speed and more. They come in a variety of platforms, ranging from mechanical trainers to smart, error-correcting technologies providing audio feedback. Drug-delivery training devices provide brand patient programs with highly realistic, but cost-effective, reusable patient training solutions compared to saline-filled syringes with real needles that may result in injury or limited learning. Their value over another training option—using the actual, medication-filled device—is obvious, especially when wastage of valuable biotech products is considered.
As the biologic and biosimilar market grows, and self-administration becomes more commonplace, more premarket and on-market brands are investing in reusable drug-delivery training devices. With biologics accounting for one-third of new medicine approvals over the last decade [4] and the emergence of biosimilars, brand teams are searching for competitive differentiators. Better training devices can be part of that differentiation.
Launch Readiness: An In-depth Overview of Training Devices and Patient Support Implementation
Since combination product development for pharmaceutical companies is an expensive, intensive and lengthy process, it’s important for brands to be able to launch once FDA clearance is granted. Brand teams need not only to roll out the drug to market, but also have the flexibility, depending on FDA review time, to have in place practitioner and patient training and educational materials. Noble’s HCP and patient training materials (Fig. 1.) include autoinjectors, prefilled syringe and respiratory drug-delivery device trainers, angle assist tools, training IFUs, smart packaging and standard packaging, printing, travel assets, and more, all based on a pharmaceutical company’s preference and patients’ feedback.
To ensure training devices and educational assets are launch-ready, a proven process has been developed to help provide continual support and guidance to clients from preproduction through postproduction. This process ensures client requirements remain on schedule via status meetings and global or country-specific strategic planning for risk mitigation, design, development and distribution.
The following is an overview of our development process for launch readiness:
• Program planning starts with gathering requirements with the brand and/or device teams, leading to a Product Requirements Definition. A scope-of-work Project Control Plan is determined by agreeing on resource commitments and development phases.
Product requirements gathering includes device analysis, existing literature review and a user needs assessment. A Requirements Summary Matrix is then developed.
• The Research and Concept Development stage includes product development research, functional value assessments, and initial design and engineering activities to develop concept architectures. A Product Development Research Report is produced based upon research in this phase, and includes user and usage profiles, and recommended concept designs. As part of best practices, the training device is engineered to emulate the real device as closely as possible to ensure patients receive a realistic representation of the functionality of the actual drug delivery device. This engineering process is more complex than with a real device because the trainer involves internal components that replicate a consistent operational sequence of a real device while also being resettable, so the patient can use the device repeatedly for training purposes.
Several steps are taken during the design and manufacturing process to ensure training devices function as closely as possible to the actual drug delivery device, such as matching external dimensions; developing internal mechanisms to accurately represent functionality and administration; and sequencing attributes, such as plunger drop speed, breakout and glide forces, sound replication, and actuation forces.
• After client review and concept revisions have been completed, a Final Requirements Definition document is created, which details product specifications and engineering activities, and provides final concept renderings for approvals. Various forms of prototyping and bench testing, as well as initial design transfer activities, occur during this phase. With launch readiness being essential for a brand, 3-D printing technologies have proven to be valuable assets allowing for rapid prototyping. Multiple iterations of drug delivery device trainer prototypes can be produced, improved upon and optimized for high-volume manufacturing.
• The Design Transfer and Prototyping phase is when major functions are analyzed for performance, various functional prototypes are produced and product tests take place to optimize the design for high-volume production. Manufacturing cost estimates are developed and submitted for client approval.
• Following the Design Transfer and Prototyping phase, production planning and tooling occurs. Some of the key activities occurring during this phase include component design review, procurement and production planning, and capacity planning. A Quality Control Plan is developed and put into effect, and assembly instructions and validation activities are determined. A detailed production schedule is developed based upon client launch requirements and pre-production samples are produced for final approval.
• The manufacturing process begins, based upon the Quality Control Plan. During this phase, client products are produced, inspected and tested. 100% verification testing takes place to ensure final quality. Besides manufacturing and quality assurance testing, other key activities include confirmation of distribution center addresses, receiving requirements, carton marks, administration of all freight documentation and final delivery to distribution centers.
• Ongoing support and guidance continues after patient training, education and support products are delivered and introduced into the market. The client services team continues working with clients on any additional support needs, including reorders, additional product development and education materials.
Click to enlarge
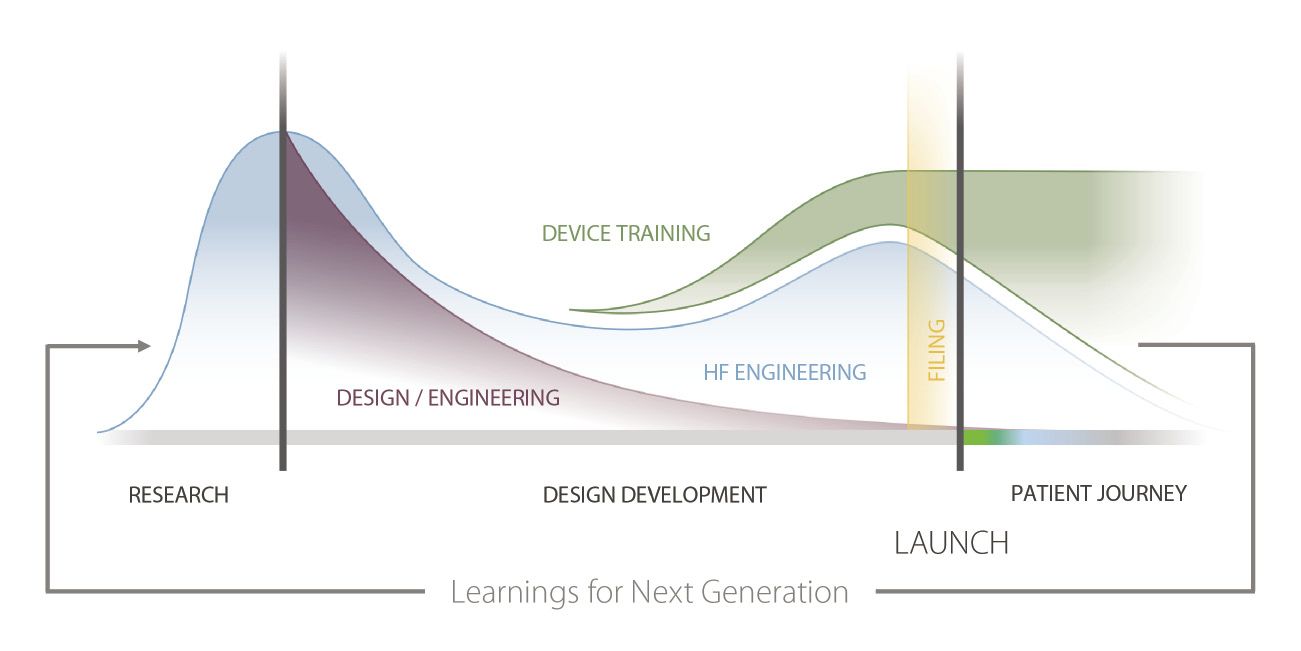
Fig. 2. By the time a product is launched, a strategy for addressing patient training should be well-established.[/caption]
Finding value in better training
As the launch process for the pharmaceutical industry evolves, there has been a shift to beginning launch strategy planning sooner (Fig. 2.). Device and commercial teams are collaborating earlier in the process, and leveraging human factor findings for refining product development and building patient-centric training and education programs to better prepare for launching in a competitive marketplace.
The use of training devices is highly beneficial for patients during the onboarding process because it allows them to more easily understand and retain training information before anxiety can build around at-home treatment. Through multisensory learning, multiple pathways are established in separate areas of the brain, resulting in a highly effective training experience and improved safety for patients. Other findings from the previously referenced Noble study found 90% of patients who used a training device rated the value of the device at a seven or higher, with 10 receiving the most selections. Additionally, 74% reported that in hindsight they would have preferred being recommended a training device when treatment was initiated. [3]
Brands that implement device trainers during the onboarding process will see higher levels of patient compliance and adherence as a result of lowered patient anxiety and an increased understanding of how to use drug delivery devices. By providing patients a better understanding of the device, with the ability to practice administration techniques as often as needed, trainers help promote positive onboarding experiences and empower patients to lead healthier lives. In the patient-centric era, companies providing reusable, device-comparable training products will be well positioned for competitive differentiation through improved patient satisfaction, adherence and outcomes.
References
- Bonds, R., Asawa, A., & Ghazi, A. (2014, November 18). Misuse of medical devices: a persistent problem in self-management of asthma and allergic disease. Retrieved from www.annallergy.org/article/S1081-1206(14)00752-2/fulltext
- Zambanini, A., Newson, R. B., Maisey, M., & Feher, M. D. (December 1999). Injection related anxiety in insulin-treated diabetes. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10624790
- Noble. (2015, November). The link between device training and patient compliance. Poster session presented at PDA - Universe of Prefilled Syringes 2015, Vienna, Austria
- Pharmaceutical Research and Manufacturers of America. (2013). 2013 Overview medicines in development: Biologics. Retrieved from http://phrma.org/sites/default/files/pdf/biologicsoverview2013.pdf
ABOUT THE AUTHOR

Paul Sullivan is the associate director of business development at Noble (Orlando, FL; www.gonoble.com), a product development company with a focus in designing and manufacturing drug delivery training and patient onboarding solutions. Prior to Noble, Paul worked at Informed Medical Communications, as a director of business development and client service and before that, as a pharmaceutical sales representative with Procter & Gamble Pharmaceuticals. He holds a Kinesiology degree with honours from the University of Western Ontario.
Save
Save