
Brand & Product Security
Latest News
Latest Videos

More News

If there’s anything we learned from the pandemic, it’s the critical need to analyze patient outcome data in real time to stay informed on the safety and efficacy of treatments in the real world

Features an ATP solution to allow for efficiency

The clock is ticking on a massive effort to bring interoperable, electronic tracking of pharma shipments in the US

A recommended checklist for manufacturers as they enlist distributor and 3PL support—and the important questions they should be asking themselves

Pressure is mounting ahead of looming unit-level serialization required by the Drug Supply Chain Security Act—necessitating a wide scope of collaboration on compliance

Outlining the work of the Open Credentialing Initiative in establishing digital proof of identity, licensure status, and authorship using digital wallets and verifiable credentials

From November 2023, the Drug Supply Chain Security Act requires healthcare manufacturers, distributors, providers, and dispensers to exchange the serialized item-level product information that has been collected, standardized, and digitized. Hitting this deadline hinges on intensive collaboration between manufacturers and their supply chain partners that must begin now


More biologic-based therapies translates into more cold chain activity. As yet, new cellular and genetic therapies are a minute part of the overall market.

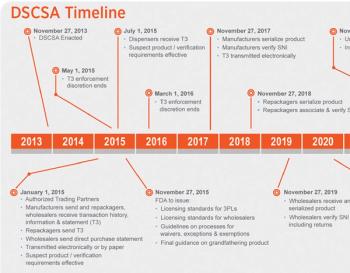
A 2017 postponement makes 2018 the key year for serializing US products; authentication makes a comeback, and cargo security goes digital

Patient support (hub) services rise in lockstep with specialty pharmaceutical launches

Drug-based therapies for opioid dependency gain acceptance and reimbursement

New industry entrants aim at expanding patient volumes even while drug compounding limits some therapy classes

Vendors and service providers scramble to serve a growing market

Pharma spends heavily to secure its supply chain, while politicians bring out the re-importation topic

More volumes of more valuable products drive growth

A silver anniversary with golden opportunities

Meeting the compliance deadlines of the Drug Supply Chain Security Act has the attention of many in biopharma product and brand security


For specialty pharmaceuticals, the connection between manufacturer, payer and patient is the rising crop of hub services


When entering the specialty pharmaceuticals arena, manufacturers need to evaluate the capabilities of their channel partners carefully

HDMA's fourth edition polling its members and manufacturers, on trends in specialty distribution














