Compliance Package of the Year goes to Merck’s Zepatier
Annual industry competition highlights the adherence benefits of unit-dose packaging
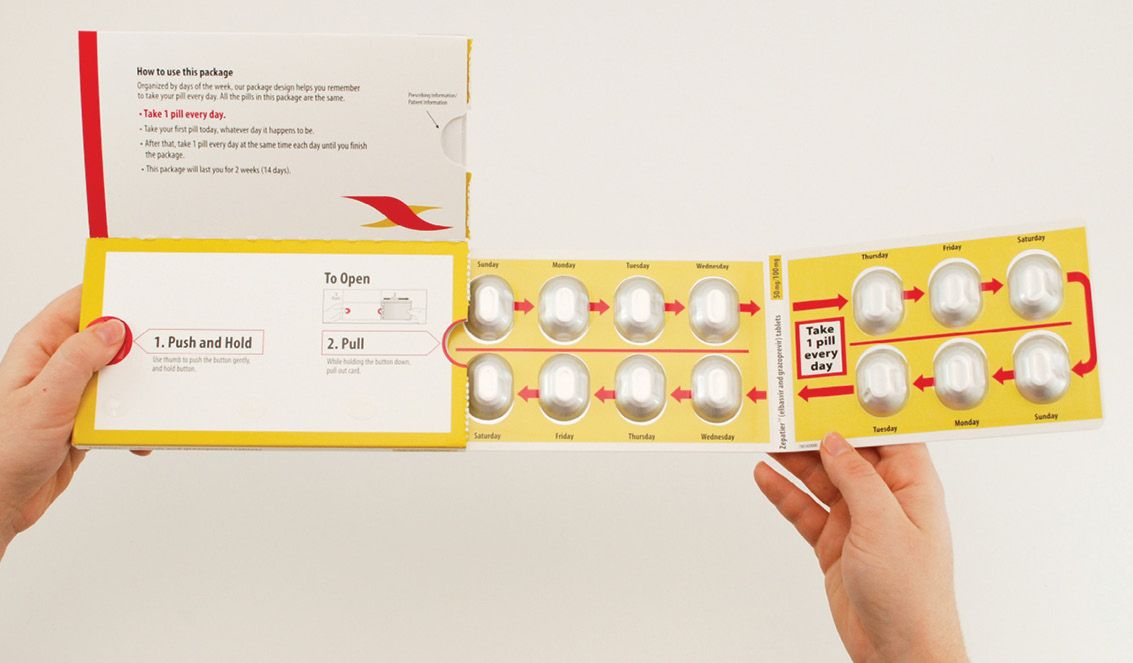
Compliance Package of the Year is Merck’s Zepatier kit
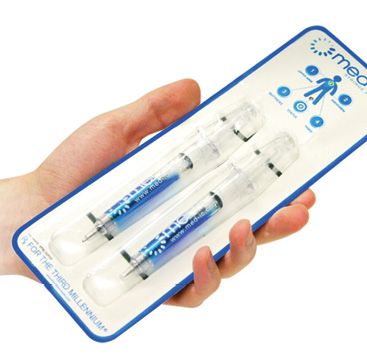
Innovative Package of the Year is Information Mediary’s Med-Ic syringe package
The winners of the
Healthcare Compliance Packaging Council’s annual Compliance Package of the Year are in: the first-place winner is Merck’s Zepatier hepatitis C treatment, featuring a medication package designed by WestRock. Other award winners:
- First Runner Up: Braeburn Pharmaceuticals’ probuphine implant kit, manufactured by Sharp Packaging Solutions
- Second Runner Up: CVS Store Brand OTC Compliance Pack
- A related competition is held for packaging innovation; this year’s winner is branded as the Med-ic ECM (Electronic Compliance Monitor), submitted by Intelligent Devices SEZC Inc. on behalf of Information Mediary Corp.
The Zepatier package promotes patient medication adherence using a custom design that integrates a calendared blister clearly labeled by day on each individual pill cavity, highlighting the two-week dosing regimen (2 two-week packages make up a monthly supply of Zepatier). Visual cues prompt medication taking and tracking. A panel in the package is devoted medication information, dosing instructions and package instructions. Product branding is clearly presented on the outer package’s readable, flat space.
Compliance Package of the Year First Runner-Up, Braeburn’s probuphine kit.

The Braeburn Pharma package is an administration aid primarily for physicians, who are tasked with placing a set of drug-eluting implants subcutaneously; the drug is a therapy for opioid addiction. The package is complex, including the implants, an implantation device and documentation necessary for regulatory compliance. The Sharp Design team partnered with Braeburn Pharmaceuticals during many stages of the product-packaging development, including design, prototype development and finally, commercial production. “The kit demonstrates the critical role that packaging and labeling can play in guiding professionals through safe and effective product use,” according to one of the HCPC judges.
Compliance Package of the Year Second Runner-Up: CVS’ convenience OTC package

The second runner-up, the CVS compliance pack, represents another win for WestRock, whose Dosepak design is employed in the packaging for a variety of OTC products. In this case, besides the readily available consumer-friendly features of the package, which contains and protects a set number of doses (14) in an easily carried carton, the container fits neatly with a merchandising format at CVS, which will put the products under the pharmacy checkout counter, making the products something of a convenience purchase by consumers.
Information Mediary’s syringe pack addresses a growing need in the biopharma industry: packaging patient-administered parenteral drugs. By packaging a pair of syringes in an electronically equipped container, the date and time of administration can be recorded; the package also provides guidance on how the dose should be administered. The package contains near-field communications technology, so that with the appropriate smartphone, an interaction can be set up between the patient and the physician. Yet another feature is the ability to monitor the temperature at which the package has been maintained—a critical quality measure for temperature-sensitive drugs.
“There is a lot of potential for on-package labeling instructions, product branding and other compliance-prompting features,” according to one of the HCPC judges.
The Healthcare Compliance Packaging Council (Richmond, VA), a group of contract packaging and related organizations, has promoted the use of unit-dose packaging (blister cards, cachets and related packaging); and for years, the pharma industry has been avid users of this form of packaging for samples and introductory product-launch kits, but then reverted to bulk packaging or 30-count bottles as products enter their adolescence. That dynamic has changed in recent years, as a continuing stream of studies sponsored by HCPC and others) point to the greater degree of adherence when patients receive their medication in unit-dose form. For one thing (and perhaps the main thing), a 30-count of pills in a calendared blister card shows the patient clearly that they’ve taken today’s dose (or doses); and how long until their current prescription runs out.
More broadly, as health systems pay attention to CMS’ scoring for improved healthcare delivery (in which patient adherence is a factor), there is the prospect that pharmacy directors at these organizations will look more favorably on adherence-enhancing factors like unit-dose packaging. Pharmacy benefit managers, likewise, might be more favorably inclined to manufacturers who include adherence features in the drugs that the PBMs put on formulary. The growth of, for example, oral oncolytics, for which better adherence to therapy is critical to successful outcomes, plays into this dynamic.
HCPC presented these awards at its annual RxAdherence meeting, held in Florham Park, NJ on May 2.
Save
Save
Save

Strategic Trends in Pharmaceutical Manufacturing for Industry Leaders
March 10th 2025This link in the pharma supply chain is undergoing a major transformation propelled by technological advancements, regulatory changes, and evolving market dynamics, requiring industry leaders to adopt innovative strategies in order to remain competitive.