Assessing Drug Pricing in Europe
How various factors are affecting the amount of funds allocated to healthcare.
Ed Schoonveld

Over the last few months, biopharmaceutical firms have been more vocal than ever about challenges in the European business environment. The likes of Bayer have openly or more discretely focused attention away from Europe in favor of the US and China. Ironically, this is happening at a time when the US is implementing a first step toward price controls through the Inflation Reduction Act (IRA).
What makes the European changes even more alarming than the disruptive changes in the US? As you probably know, the European single-market government payers have been able to extract prices for drugs that can be significantly lower than in free-market systems such as the US. Whether you believe that US prices are too high or not, the fact is they are in general higher, suggesting that patients who live in a free-market system for prescription drugs are subsidizing governments and patients in single-payer monopsonies.
European governments are struggling to make ends meet due to the economic recession following COVID-19, along with the impact of the war in the Ukraine. Let’s look at some of the specific changes.
Germany: Sometimes coined as “AMNOG 2.0,” the German government has significantly tightened the evidence requirements for orphan drugs, essentially requiring a major or considerable benefit demonstration for commercial viability. In addition, it introduced discounts for combination drugs, shortening of the free pricing period from one year to six months, and an increase of the mandated rebate to 19% for all drugs.
France: Over the last decade, France has systematically extracted rebates for prescription drugs, so that the net drug budget has declined by 4.5% per year since 2011.
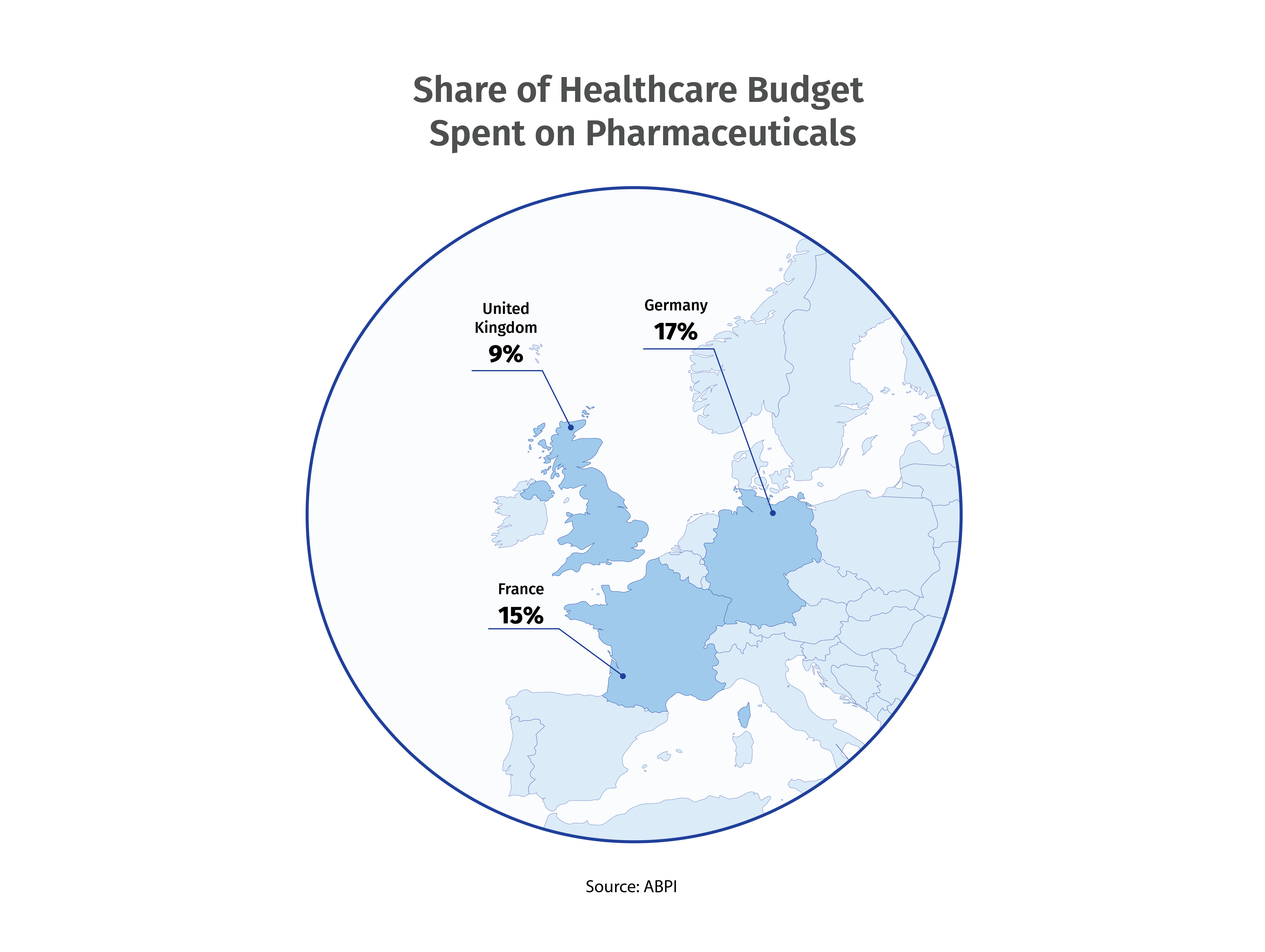
UK: Due to overspending of the National Health Service (NHS) on drugs, the government wants to recoup 24.4% as a rebate, per the voluntary scheme for branded medicines pricing and access. This comes on top of a decline in willingness to pay by lack of consumer price index adjustment of cost-effectiveness thresholds since 1999. The Association of the British Pharmaceutical Industry notes that the share of healthcare budget spent on pharmaceuticals is only 9% in the UK, versus 15% in France and 17% in Germany.
Spain: Has introduced a more formal cost-effectiveness criteria as part of national price approval. In an initial assessment, this can have a large impact on the number of drugs that will be viable for launch.
The European Commission is contemplating legislation that will reduce effective patent lives from 10 to eight years and/or make it mandatory for manufacturers to commercially launch their approved drugs in all EU member states, irrespective of the locally determined price. This is really concerning, since a mandatory launch at the lower range of European prices will quickly cascade throughout the region.
What does this all mean for biopharma? Should companies de-emphasize Europe as some have done? That depends. For products with strong evidence of patient benefit, there may still be a strong case to launch in Europe. In cases where the benefit is minimal, or where the company has not been willing to develop the required evidence, commercialization will be even less successful than it already was prior to these changes.
Interestingly, US IRA-related changes may make the hurdle to be prepared for European access needs less high. Instituting a timeclock to the Centers for Medicare and Medicaid Services (CMS) negotiations for every new drug may motivate companies to develop stronger evidence and prioritize larger indications before formal launch, as it will result in more rapid uptake and higher cumulative revenue until CMS price control kicks in.
Every situation is different; however, this is another indication that the biopharma industry needs to engage in more detailed global trade-off analyses that are well informed by a strong understanding of how evidence scenarios result in access and product utilization in the largest global markets. Many companies may still have some homework to do in order to achieve this and go beyond lip service in delivering value.
About the Author
Ed Schoonveld is a value and access consultant, and author of The Price of Global Health.
