Pharma traceability: Marching toward 2023 go-live
FDA-accepted pilot projects hope to demonstrate how industry compliance could be realized by the 2023 deadline
Fig. 1. Sharp Packaging recently expanded its vial labeling and cold sterilization capacity in Allentown, PA, with a view toward combining serialized product and personalized medicine. Credit: Sharp

The Haitian proverb, Dèyè mòn, gen mòn (Beyond mountains, more mountains) comes to mind when surveying the current scene in pharma traceability. The industry has been on a long slog to get appropriately barcoded labels on each saleable unit of products—the base-level requirement of the 2013 Drug Supply Chain Security Act (DSCSA). Now, each year through 2023 will have a milestone or deadline for bringing a digital, end-to-end traceable system for products to life.
Growing their businesses
The current target is “saleable returns”—the products, still suitable for consumption, that are returned to wholesalers or manufacturers from pharmacies and other downstream trading partners, amounting to 2-3% of overall US commercial distribution. Under DSCSA, by November 2019 wholesalers are obligated to verify the authenticity of returned products before sending them back into distribution, and this obligates manufacturers to have a resource to respond to wholesaler queries. That obligation, in turn, has spawned the current scramble for verification router services (VRSs) offered by numerous traceability vendors.
Soon, pharmacies and hospitals will need to document the identify and products they receive, and after that, to confirm their authenticity. By 2023, systems that track product movements electronically will need to be up and running. Along the way, a national database of state-licensed distributors will need to be realized, and repackagers, secondary wholesalers and other supply chain participants will need to have their systems in place.
“The pharma industry might think that it’s done, now that nearly all products are being labeled with the required serial codes,” notes Greg Cathcart, CEO of consulting firm Excellis Health Solutions. “But that represents about 20% of what will ultimately be necessary for full DSCSA compliance. Warehouse management, trading partner communication and overall supply chain interoperability will still need to be tackled.”
To be sure, there are numerous refinements or upgrades going on with the packaging and labeling technology that has been installed to date. A particular area of activity is aggregation—the step to precisely identify which barcoded items are in a (barcoded) case, after the initial serialization.
Many manufacturers, noting that aggregation was not a DSCSA requirement, skipped or postponed aggregation implementation; now the reality that wholesalers by and large will not accept un-aggregated cases has set in. “We’re got a lot of people approaching us who weren’t expecting to aggregate,” says Andrew Pietrangelo, president of Antares Vision North America. The company has developed three levels of aggregation—manual, semi-automatic and fully automatic—to align with the volume or pace of packaging going on at a facility. Pietrangelo, while noting that Antares now offers both hardware and software for DSCSA compliance, says that the company has expended considerable effort to make its hardware integrate well with other vendors’ traceability technology.
Aggregation and—of equal importance—disaggregation are vital elements of managing pharma warehouses, and the distribution centers of wholesaler/distributors. There is growing activity among the traceability vendors to provide either warehouse management systems that incorporate the aggregation of serial data, or so-called “edge solutions” that speed the collection and validation of serial data during pick-and-place operations. A couple years ago, TraceLink, a leading traceability vendor, acquired Roc-IT Systems for this purpose; there are competing offerings from DMLogic; Antares, Optel Vision, Acsis and others.
Barcoding itself is still a concern: recent surveys by GS1 and HDA have shown that a small but noticeable percentage of serialized product either have incorrect coding, or have legibility problems. Tim Kearns, sales manager at Videojet (a provider of multiple types of marking technology), says that “To date, the pattern has been that serialization project managers have tended to treat the marking technology as an afterthought. Substrates, line speeds and other factors can scramble the marking process.” Often, the fix is simple—choosing the appropriate ink, or ensuring the cleanliness of the packaging line environment.
Kearns adds that there’s attention currently being paid to how cases are marked (which, in fact, can be part of the aggregation process). The company has developed a label printer applicator, such that a case’s label is being printed as it is being applied to the case. The advantage is that the information on the label (which is tied to the serial codes actually in the case) can be verified as the label is applied; if a package is not included in the case as it gets filled, that change can be noted as the case label is printed. Overall, the technology allows for higher accuracy and faster case-packing speed.
VRS challenges
Much of the discussion going on in DSCSA circles this year involves the Verification Router Service, which was defined, more or less, by a pilot project of the Healthcare Distribution Alliance in 2017; now the distributors are racing to prepare for the nominal November 2019 deadline for having a functioning process for verifying saleable returns prior to re-introducing them into distribution (as DSCSA requires). There is a good chance that implementation will be delayed, given that a fully functioning system will require millisecond response from the IT system of a wholesaler, through the routing service, to the manufacturer, and back.
An HDA task force devoted to VRS implementation developed specifications for how verification messages could be quickly transmitted from requestors to responders (generally, a manufacturer); these specifications were shifted over to the GS1 organization and formalized as the GS1 Light Messaging Standard (LMS), approved in January.*
Nearly every traceability vendor has now developed a VRS, often as a module to be attached to their Level 4 traceability-management system. Some of them are offered as a free extension of their system; others add a charge. TraceLink, which worked with HDA and GS1 to develop open standards for LMS, says that it is deploying its Product Information Manager as its VRS solution, according to Brian Daleiden, VP, industry marketing and community. Adents, another vendor with a Level 4 system (Prodigi), announced a partnership in May with VAI, a provider of ERP systems for managing pharma warehouses (among other industries).
A functioning, industry-wide saleable-returns process will require each VRS to interoperate with each other one; there has been testing among the traceability vendors to ensure that their systems work together, but there is also dissension over how well this is working. “There is a lot of confusion in the market, with vendors trying to lock down their customers over how to implement the VRS,” says Julien Faury, VP of operations at Adents.
VRS wasn’t conceived as a mini-version of a complete, end-to-end supply chain network for the pharma industry, but in fact, that is how it is playing out currently. “VRS has become much bigger than people realized initially,” notes Perry Fri, HDA’s EVP for industry relations, membership and education.
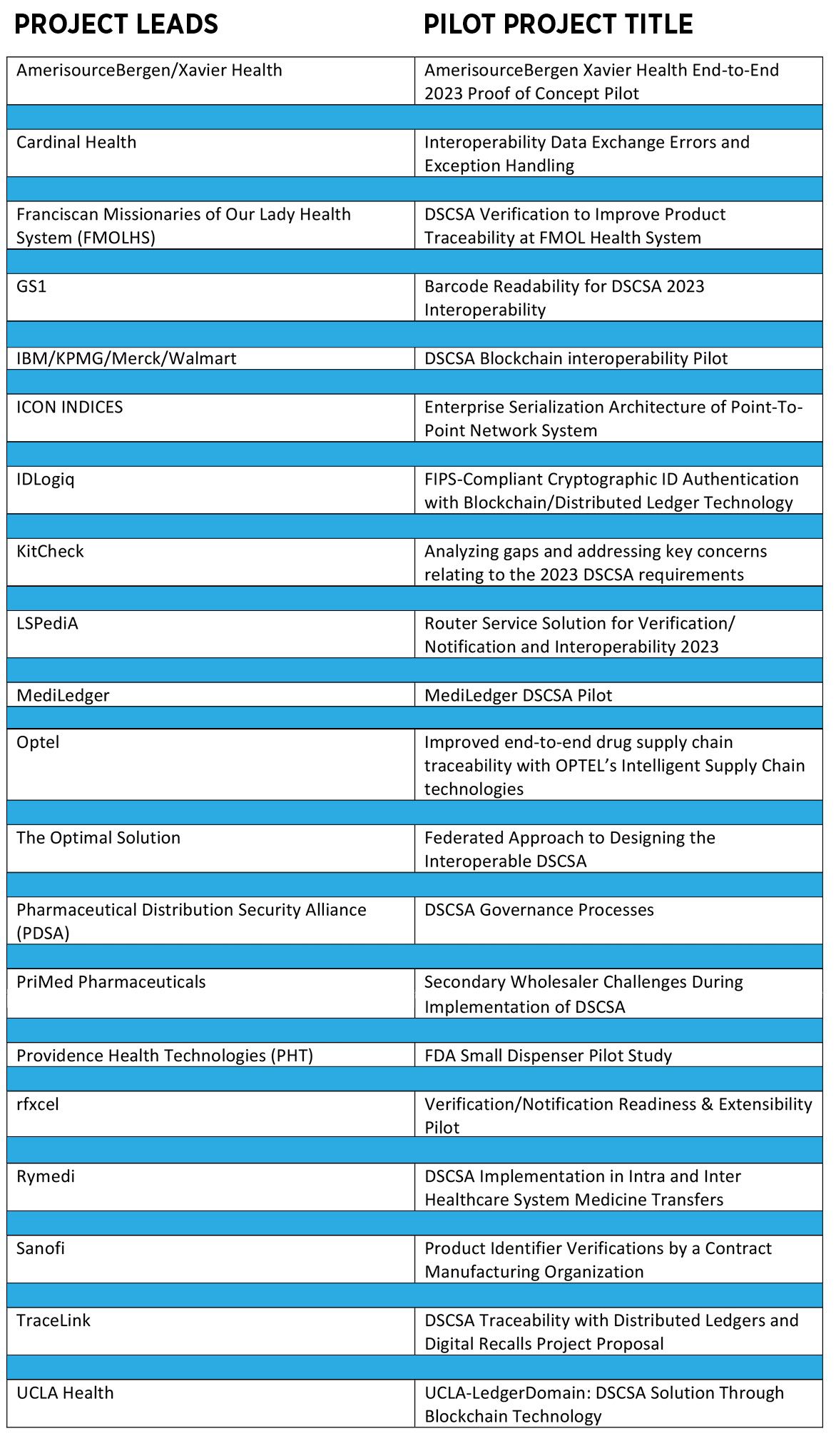
Fig. 2. FDA’s list of accepted DSCSA pilot projects.
Learning from pilots
Confirming this importance, VRS is the subject of several of the pilot projects accepted by FDA in April, the result of an FDA request for proposals earlier in the year (see table). The list of projects demonstrates a desire by FDA to bring in a broad range of pharma supply-chain participants, from manufacturers to distributors to health system pharmacies, large and small companies, and vendors along with their clients. Nominally, the projects are supposed to run through the rest of this year, with FDA collecting reports and publishing results early next year.
Some of the projects represent a mini-network of their own: the Optimal Solution project will involve not only Systech, a longtime serialization and traceability vendor, but also RxTransparent, FarmaTrust, T-Systems, Cryptowerk and CalQLogic, and will encompass blockchain technology as well as Systech’s e-Fingerprint authentication technology. “We believe we are creating a unique comprehensive solution with an unmatched combination of partners,” stated Ara Ohanian, CEO of Systech, when the pilot was announced.
Another interesting collaboration is IBM/KPMG/Walmart, also showcasing blockchain technology. IBM has been working with Walmart on a blockchain-based food-sourcing network for several years; the company also has an ongoing relationship with KPMG to develop business applications based on IBM Watson, its “cognitive” technology for managing data. (Old-timers can remember IBM’s earlier involvement in pharma traceability, a project that was eventually sold off to a company that was subsequently acquired by rfXcel.)
Blockchain buzz
The FDA pilot list is notable for the number of blockchain-based projects; a year ago, the combination of blockchain and DSCSA compliance was looked on with considerable skepticism; now, it could be argued that it is the future direction of DSCSA compliance.
At this point, there are two solid benefits for blockchain (or, in a broader business-software context, distributed-ledger technology), and one serious detriment. The benefits are that information is inherently preserved and protected by the blockchain; each member (“node”) of the chain is supposed to keep a record of all transactions passing along the network, and to synchronize this information in diverse locations, thus preventing an overwriting in one location corrupting all the other locations. The second benefit is that, with the right credentialing process, only approved or accepted participants can upload or download information (this is the “private” form of the chain). The one obstacle to blockchain implementation in this application is that it is slow, and becomes slower the more data traffic flows through the network; transactions will need to be recorded and executed rapidly, given the tens of thousands of potential participants.
MediLedger, a startup initiative now part of a company, Chronicled, has garnered the participation of three of the top US wholesalers, along with a number of pharma companies, to demonstrate its capability. Chronicled brings a technique called “zero knowledge cryptography” to blockchain communications that enables a high degree of secure privacy to transactions. The company is also notable for receiving $16 million in funding at the beginning of this year to build out its application.
Another blockchain pilot on the FDA list comes from TraceLink, which is something of a turnabout from how it looked on the technology in the recent past. TraceLink’s Daleiden says that the company has had internal teams evaluating blockchain technology for a couple years, and that “we’re not jumping in with both feet today; we’re looking at the pilot as a proof of concept.” TraceLink’s initiative is being called Trace Histories, and is designed to provide a way to preserve the information that is currently contained in a document defined by DSCSA called Transaction History; this document is supposed to sunset by 2023 when DSCSA is fully operational. Trace Histories is not yet a formal TraceLink product.
Blockchain also figures in the Systech (Optimal Solution) pilot; Systech has been working with a UK company called FarmaTrust previously. Adents has been working with Microsoft on its own blockchain implementation.
“One thing is for sure about blockchain,” says Daleiden, “there will not be one solution used by all participants; it will be a heterogeneous environment. So, whatever blockchain technology is employed, it will have to be interoperable.”
Governance
The FDA pilot list also includes something of an outlier: the Pharmaceutical Distribution Security Alliance (PDSA). The “pilot” here appears to be an ongoing effort, announced earlier this year, to develop an industry-wide consortium to develop a consensus over how DSCSA will eventually be operationalized. There are many issues around DSCSA that require a degree of collaboration among pharma supply-chain participants, even organizations that in some ways are on opposite sides of the negotiating table (for example, PBM-run pharmacies and manufacturers). PDSA was in operation in the early 2010s as negotiations intensified between the private sector and FDA as DSCSA itself came into being; it never shut down subsequently, according to an executive involved in its revival, but is now becoming a valued clearinghouse for cross-industry collaboration.
Current membership in the group includes nearly 20 pharma companies (including biotech, pharma and generic), and a host of trade associations, including PhRMA, HDA, NACDS, BIO and the Assn. for Accessible Medicines (the former Generic Pharmaceutical Mfrs. Assn.)
PDSA issued a pair of position statements in April (available on its website, pdsaonline.com, at https://pdsaonline.org/white-paper-download/). According to the executive source, a workshop was held in early May, and a proposal for how a board will be constituted, and how the alliance is to be funded, are being reviewed now. A decision will be announced in the fall for how the group will go forward.
Anti-counterfeiting revives
Although beyond the scope of DSCSA, there seems to be growing interest in investing in overt and covert anti-counterfeiting technologies in pharma today. The topic has actually never gone away, but lost some momentum in the aftermath of the passage of DSCSA. Advocates of the technology point out that serialization is not a bulletproof defense against counterfeits, especially when ex-US markets are considered; it also provides a belt-and-suspenders approach to product integrity regardless of geography.
For several years now, Systech International has touted its e-Fingerprint technology, which depends on storing details of the physical attributes of a label (which are enough to provide a unique “fingerprint”); when the image of the label in the field is compared to a stored version, the authentication is confirmed. The company says that it is having success in non-pharma applications, while continuing to offer it to the healthcare industry.
This spring, Covectra, a traceability software provider, announced an alliance with VerifyMe, a provider of brand and identity protection technologies, including covert inks. “The recent mandate by the FDA for serialization, track and trace technology for the pharmaceutical industry is a ripe multi-billion-dollar opportunity for our partnership to address,” said Stephen Wood, Covectra CEO, when the partnership was announced. A couple years earlier, Covectra had introduced StellaGuard, a technology that combines random markings with 2D barcode information.
Meanwhile, the printing developer Videojet has an ongoing relationship with Applied DNA Sciences, which develops unique tags based on DNA strings; the tags are added into inks that Videojet provides with its machines. In a similar fashion, TruTag Technologies has been marketing a silica-based tag, based on spectral patterns, to uniquely identify packages and products. The inert silica is capable of being incorporated into pill or tablet formulations as well.
These and other technologies are finding applications in food, consumer goods and multiple other applications; and in a similar fashion, the serialization/traceability vendors that have been chasing business with the global pharma industry for years are now branching out into other markets. “As a group, these vendors have developed a lot of industry knowledge over the past five years,” says Antares’ Pietrangelo. “Now we can apply this proven knowledge downstream and elsewhere.”
