The under-regulated world of patient services
Pharma needs better internal policies to manage its increasing patient interaction
Fig. 1. At least four federal laws, plus regulations on adverse-event reporting, can impact the conduct of a patient support program. Credit: Helio Health

It’s axiomatic that
the trend toward specialty pharmaceuticals is expanding the range of patient-oriented services that the pharma industry is involved in: the very definition of a specialty pharmaceutical includes ones that require patient support. This has also been one of the drivers of outsourced hub services—hiring independent providers to help navigate the prior authorization process, reimbursement support, nursing and other health maintenance activities.
But, according to a survey recently conducted by Helio Health (Hoboken, NJ), there is trouble brewing in the patient support world: a lack of well-understood operating standards is creating dramatic risks in violating a variety of federal laws and regulations, including anti-kickback statutes, False Claims Act and HIPAA patient-privacy violations (Fig 1). The topic received a jolt of attention this summer when United Therapeutics announced a $210-million set-aside to address a potential DoJ settlement over its copay assistance programs. “We expect any such settlement will include a settlement payment to the government, and it may also include non-monetary obligations, such as our entering into a corporate integrity agreement (CIA),” the company wrote in its latest 10-Q statement.
United Therapeutics’ plight is certainly not the first such enforcement action, and it’s very likely not to be the last, given some of the Helio Health findings: While two-thirds of the respondents could identify an individual responsible for compliance around patient services, the other third could not. Fewer than half of patient support teams had staff using call scripts when engaging with patients (which could imply improper communications); 19% of respondents also noted that patient services support could be performed for off-label situations.
Fig. 2. While 40% of manufacturers have a data firewall between sales reps and patients, others have no barrier or have indefinite restrictions
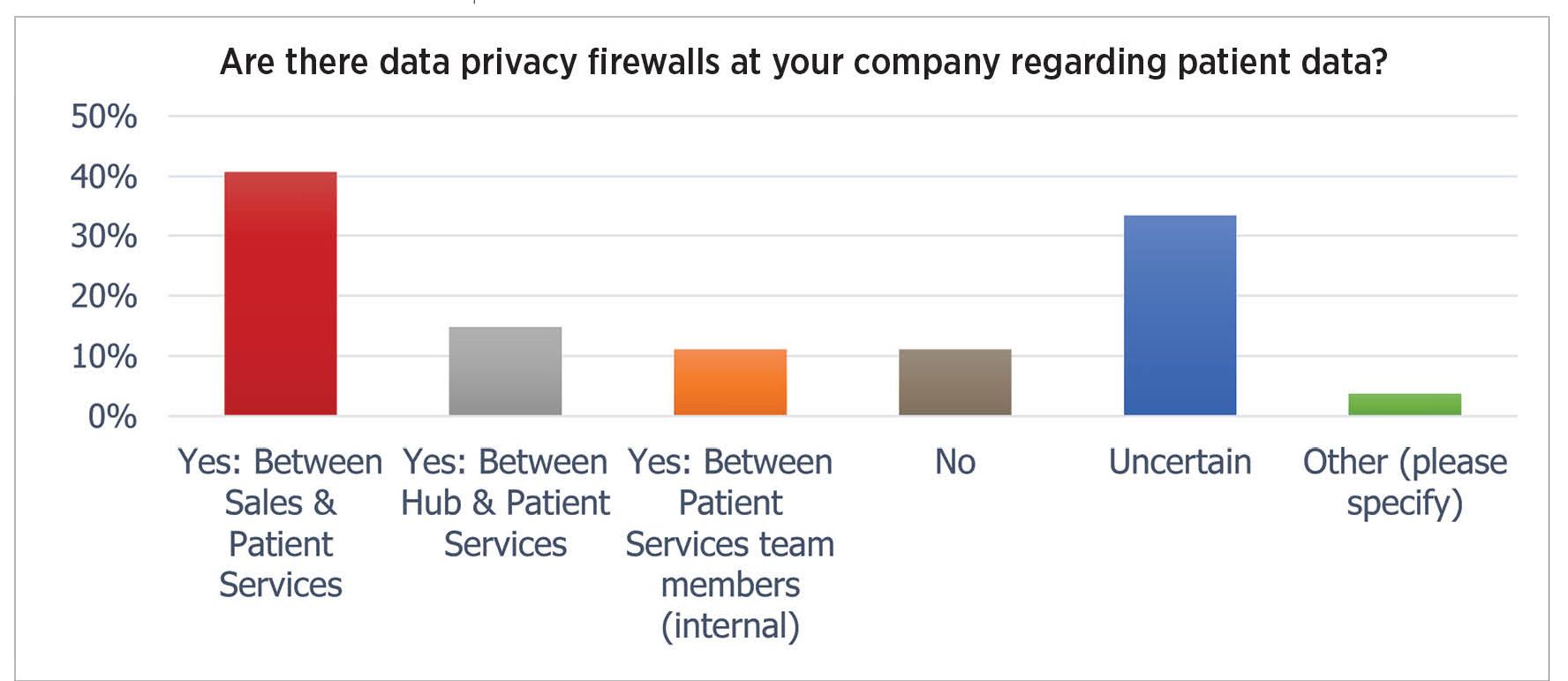
Patient privacy is an area rife with violation risks. Federal rules limit who can have access to patient data; the rules extend to “business associates” who in turn must have their own protections in place. But the survey found that 17% of respondents allows patient data to be seen by sales reps, and 10% of respondents did not have privacy firewalls in place, while a further third were “uncertain” about the firewalls (Fig. 2).
“People expect black-and-white rules for these patient support activities, but they find that there are instead many gray areas,” notes Manny Tzavlakis, managing partner at Helio, adding that the worst situation is to leave these regulatory areas unaddressed by internal policies. “On a 0–100 scale of nonexistent to full readiness for managing patient support programs, I’d say the industry is around 10–15.”
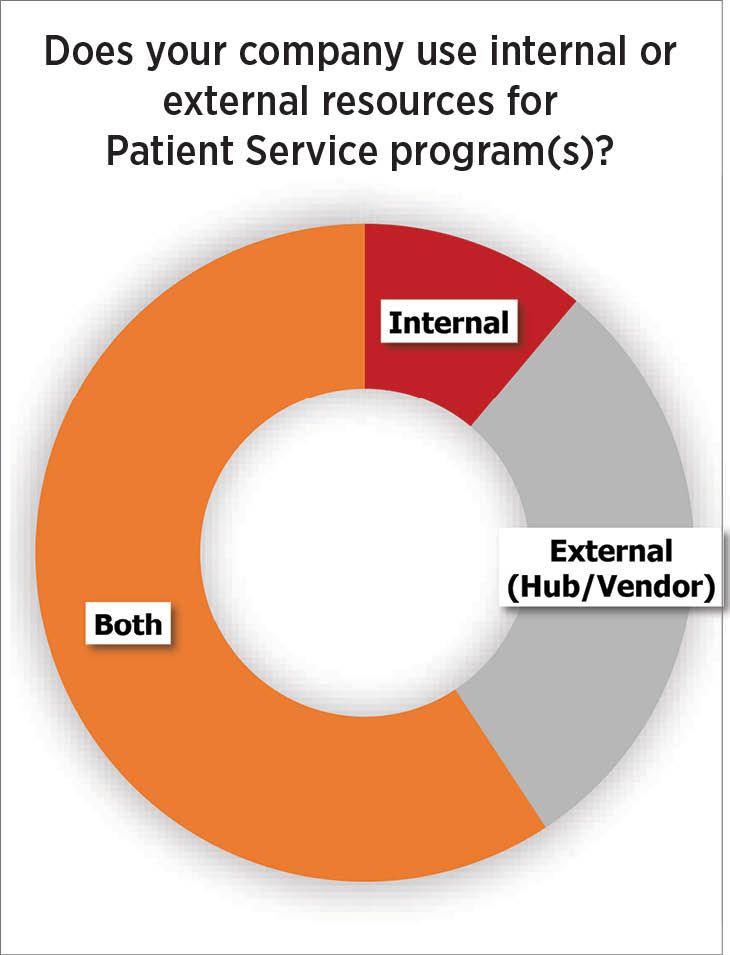
Fig. 3. “External resources” (outsourced hub programs) are used partially or wholly by manufacturers for 89% of patient support programs
Agg spending days
Tzavlakis, who worked in the past as a senior partner at Huron Consulting in life sciences compliance, likens the situation to the early days of aggregate-spend reporting, where industry downplayed reporting requirements and ignored third-party payments. “Now all this is regularly reported in the annual Open Payments system; industry put in policies and made this something of a routine.” By instituting internal practices and safeguards—and auditing or monitoring the resulting activity—the problems could be reduced.
All this is happening while the industry is still resolving how deeply it wants to be involved in patient support. The Helio survey shows (Fig. 3) that just under a third of respondents use an external hub services provider only, while another 11% only handle it through internal staff, and the majority (nearly 60%) do both. Whether internal or external, patient support teams now include call center personnel, reimbursement specialists, nurse educators and case managers. Just over 40% of companies site their patient support teams within a brand or commercial team.