Brand enhancement and safety with unit-dose packaging
Packaging designers continue the uphill battle for converting the US pharma industry to unit-dose, compliance-improving packaging
Unit-dose packaging—blister cards or strips, pouches, sticks, or certain types of vials and prefilled syringes—is taking on a new emphasis in an era where drug packages are going to be traced from place of manufacture to place of dispensing in the US, and in much of the rest of the world. Traditionally, most oral solids (pills, capsules) are packaged in bottles of 30- or 90-counts, especially for chronic care. But in Europe and elsewhere, unit-dose packaging takes priority. There are technological reasons to prefer unit-dose packaging in Europe; there are business reasons to prefer bottles in the US.
With passage of the Drug Supply Chain Security Act in 2013—and with the deadline for manufacturers having a unique serial code on each package (whether in bottle or unit-dose form) by November 2017, the scale tips somewhat more in the direction of unit-dose packaging, according to Walter Berghahn, executive director of the Healthcare Compliance Packaging Council (Bon Air, VA). “The problem with serialization in the US is that, for many bulk oral-solid deliveries, you lose the serialization information at the pharmacy, where the drug gets dispensed into generic yellow pill bottles. So, in the context of extending supply chain security all the way to the patient—which was supposed to be the goal of that law—drug identification can be lost.”
To a degree, this depends on how methodical local pharmacies are in storing data on their drug inventories—something that the US retail pharmacy business is required to do, but which is coming into force slowly—but Berghahn’s point is that supply chain security all the way into the patient’s home medicine cabinet has an extra hurdle to overcome. In a related context, he points out that the crisis over the distribution of opioid-based drugs, which are prone to excessive abuse and have become a subject of national concern, could partially be addressed by having those drugs dispensed in unit-dose form. “Serialized unit doses of these drugs would provide law enforcement more opportunities to trace their origin, and could address the issue of counterfeit versions of the pills currently showing up on the street,” he says.
HCPC’s historic emphasis (the organization is celebrating its 25th anniversary this year) has been on the benefits of unit-dose packaging to improve patient adherence and compliance with therapeutic regimens (hence the term “compliance packaging”), and here, too, there are developments that are tipping the scales more in unit-dose packaging’s favor. Insurers and their pharmacy benefit managers (PBMs) want better adherence in order to minimize the cost of healthcare; when patients take their medications as prescribed, there should be fewer hospital visits or reduced disease progression, especially with the high cost of specialized therapeutics today (to cite one obvious example: if a hepatitis C patient does not stick to the 12-week regiment for the latest treatments, the $50—$100-thousand cost of the regimen goes for naught).
Healthcare providers have always wanted patients to stick to their therapies; now they have incentives, under HHS guidelines, to record adherence rates and to reduce return visits to hospitals. Manufacturers, of course, have always encouraged better adherence at least as a policy stance; now some of them are investing in compliance packaging to improve overall adherence rates, and using that investment to justify better formulary placement with PBMs.
“Unit-dose packaging is well positioned to address a part of this concern by providing a package that dispenses one tablet at a time and in some cases shows an indication of when a medication should be consumed,” says Anna Frolova-Levi, VP & director of sales and marketing at Bilcare Research. “While the pharmaceutical industry has been experiencing many changes in the last decade, it is entering a period where more attention is paid to the final patient outcome.” Bilcare, with manufacturing and service units around the globe, provides films and foils for unit-dose packaging, as well as manufacturing of clinical trial materials including packaging.
Fig. 1. MannKind’s Afrezza carton has inhalation devices as well as blister-carded drug cartridges. Credit: PCI
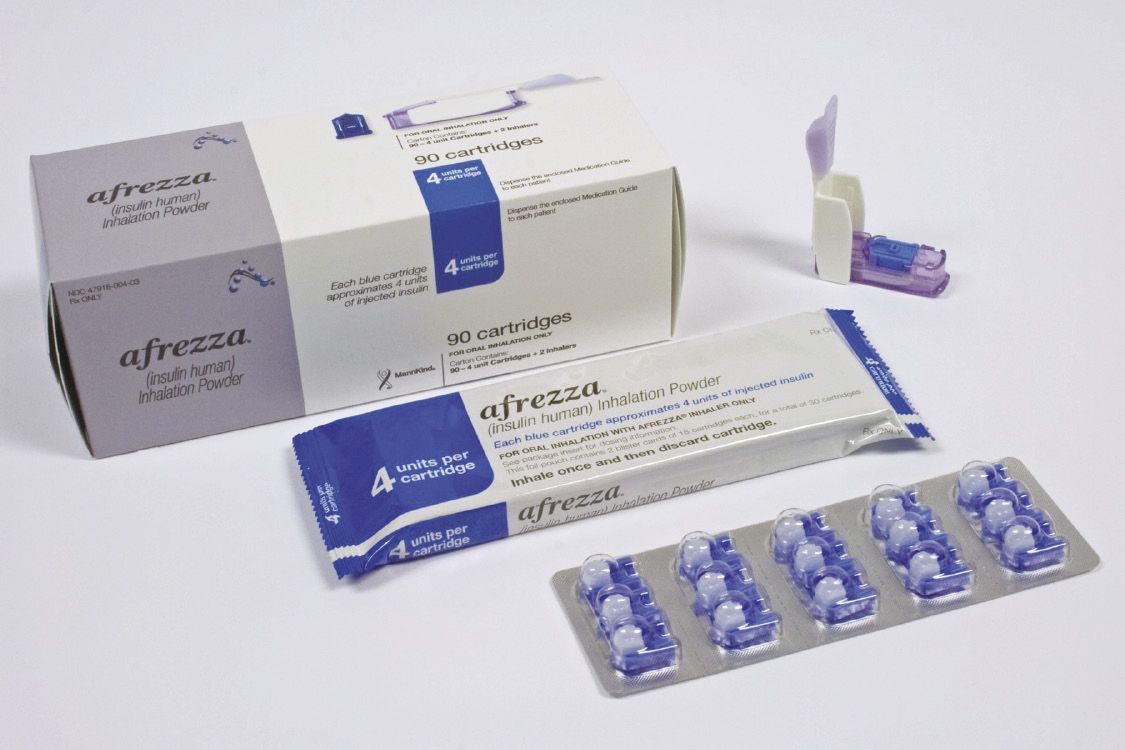
“As a general rule, there’s a market trend toward specialized drugs of high value for rare diseases, oncology and the like,” notes Justin Schroeder, communications manager at PCI (Packaging Coordinators Inc., Philadelphia, PA). “This trend, combined with the select patient populations for these drugs, reinforces the demand for better and more effective packaging that improves adherence.” PCI, now with operations in both the US and Europe, provides contract packaging and contract manufacturing of oral solids; a relatively new clinical trial services unit is building capacity.
PCI has a chance to demonstrate these principles with its work on the Freeze (inhaled insulin) product—although the long-term outcome of that is in doubt. Afrezza was approved, for MannKind Corp. (Valencia, CA) in 2014, which then entered into a commercialization agreement with Sanofi-Aventis, but at the beginning of this year, based on poor sales, Sanofi backed out of the agreement. As of presstime, MannKind remains committed to keeping the product on the market, citing the enthusiasm of some patients for a non-injectable form of insulin for diabetes care.
The Afrezza kit comprises a pair of inhalers (each meant to be used for 15 days) and four boxes of blister-carded “pods” that contain the powdered, inhalable insulin. The blister cards not only enable patients to keep track of usage, but, because they contain different amounts of insulin, can be combined to give a variety of dosages depending on patients’ need. All of this is color-coded for ease of use.
“It’s a coordinated approach,” notes Schroeder. “PCI didn’t make the inhaler or pods, but we’ve been responsible for the overall packaging and kit assembly.” PCI has submitted this product as a contender for HCPC’s Annual Compliance Package of the Year, set to be announced just after presstime.
It has been a fairly conventional practice to use compliance packaging with therapy regimens that involve a strict schedule of administration for effectiveness (such as birth-control pills) and with regimens that involve a variation from day to day or week to week of dosage strengths (also a feature of birth control as well as other therapies). Those applications, as well as the starter or sample kits offered at launch by manufacturers, have been the bread-and-butter applications of unit-dose packaging in the US in recent years.
The enhanced attention being paid to adherence for chronic therapies is attracting manufacturers to “calendarized” packaging, which gives patients an easy-to-observe pattern of pill counts over the course of a month. Blister cards color-coded by week of the month or with simple day-by-day counts (pill 1, pill 2, etc.) can do the trick. WestRock (the merger of MeadWestvaco and RockTenn, finalized in mid-2015) announced its latest calendarization package in February, the PerfPak, which features a multi-day calendarization layout, along with child-resistant security features for each pill cavity (as opposed to alternative designs that provide security for the entire blister card within a “wallet” container).
Fig. 2. Blister cards from CSP Technologies feature oxygen and/or moisture barriers. Credit: CSP
Clinical trial packaging
One of the more hospitable situations for unit-dose packaging is in clinical trials, where, typically, limited production runs of a product being developed are used, and where careful control of environmental conditions are maintained. PCI’s Schroeder, whose company has invested substantially recently in clinical trial packaging, says that unit-dose packaging is more popular than in commercial packaging, in part because the patient experience of the drug is more controllable. “Drug developers go through amazing gyrations to mask the trial drug’s identity from the comparator drug,” citing the example that if pills in a bottle don’t have the same shaking sound, it can create a problem for the trial. Additional features to be matched are the color and smell of the pills, the paperboard used for cartons, and, in the case of vials, ensuring that the liquid appears the same under refrigerated and room-temperature conditions. “All this is even more important in crossover trials, when the same patient starts on, say, the comparator drug but then switches over to the trial drug. All sensory characteristics need to be the same.”
PCI, like a few other leading contract manufacturers, is positioning itself to be a one-stop shop, from drug development to commercial manufacturing. Still, he notes in a unit-dose context, the frustration of seeing “a drug in unit-dose packaging during trials, and then bulk packaging when it’s commercialized.”
Zeroing in on stability
Berghahn’s HCPC has another rabbit to pull out of its hat for drawing attention to unit-dose packaging: shelf stability of oral solids. It has long been known, although only loosely attended to by FDA, that pills in bottles in patients’ homes can degrade over time; for this reason, some pill bottles include a desiccant module to reduce humidity (as one several countermeasures available). Last year, HCPC published a study* evaluating the physical properties of three common drugs (simvastatin, lisinopril and metformin) when subjected to elevated relative humidity and/or elevated temperature. While cautioning that the results were not definitive on the long-term efficacy of the drugs, Berghahn said that “We feel additional research should be conducted to determine whether current packaging formats are effectively protecting the wide variety of drug products on the market, each with unique environmental sensitivities, as they relate to moisture and oxygen ingress and exposure during ‘in-use’ conditions.”
The study looked at three packaging materials: polypropylene (typically used in amber bottles at pharmacies); high-density polyethylene (typical for white pill bottles) and polyvinyl chloride film (typical for the basic blister packaging in long-term care settings). By contrast, packaging suppliers such as Bilcare, Klöckner Pentaplast, Honeywell and others have been offering high-barrier films that resist the intrusion of water vapor or oxygen; and foil film providers like Constantia Hueck offer aluminum film or aluminum-containing multilayer films not only for ease of opening the pill cavity but also to provide a water vapor barrier.
These barrier films aren’t new by any means; Honeywell has been offering what is arguably the best known film, Aclar (chemically, polychlorotrifluoroethylene, or PCTFE) for years to the pharma industry; what the HCPC study does is draw attention to an apparent gap in drug stability—what happens after it has been dispensed by the pharmacy, and sits in the patients’ homes for upwards of three months. Berghahn says that an updated version of the 2015 study is about to be published by the US Pharmacopeia organization, to draw further attention to the issue among pharmaceutical scientists.
Packaging developers are moving the debate yet one step deeper into packaging science with the development of computer programs to analyze and predict barrier films’ performance, and to provide meaningful design information even while drugs are in the clinical research stage. Bilcare has been offering the BilcareOptima service for several years now, providing what it says is the first instance of packaging design via FDA’s Quality by Design standards (QbD generally is intended to predict performance before manufacturing machinery and processes are specified in FDA approval documents).
“Tests that determine the optimum blister film barrier properties for packaging certain pharmaceutical products can take as little as 15 business days,” says Bilcare’s Frolova-Levi. “This sort of expedited drug stability testing helps packaging engineers and formulators at pharmaceutical companies overcome the time and resource crunch they face when attempting to achieve successful stability studies.” And it not only predicts needed packaging requirements ahead of time; it can also preclude expensive “over-packaging” or use of unnecessarily expensive materials, she adds.
Klöckner Pentaplast, meanwhile, opened what it calls the “kp i.center” in Charlottesville, VA, a year ago, to provide a pilot and testing facility for packaging clients to work with Klöckner experts on packaging prototypes. The research center makes use of software and expertise developed by the company, called the BlisterPro XCEL Suite of design services. And that system, in turn, incorporates technology acquired by the company a couple years ago from FreeThink Technologies, called ASAPrime shelf life modeling software and ASAP laboratory studies.
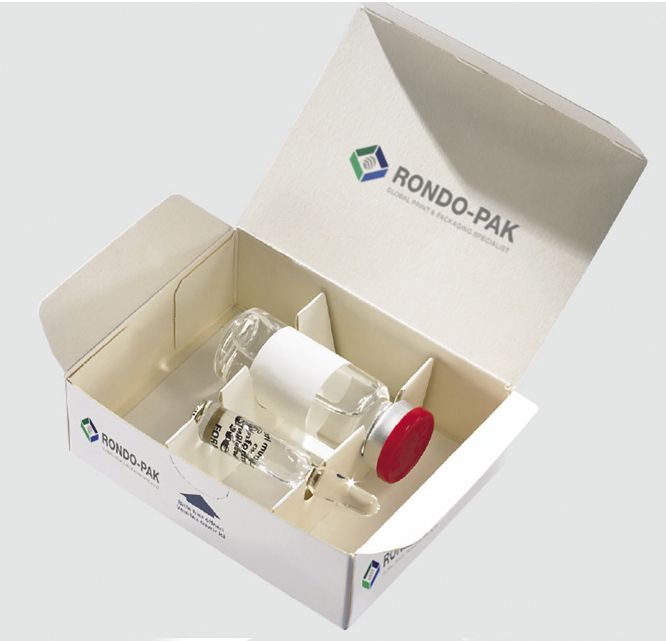
Rondo-Pak has introduced a ‘quick to market’ vial assembly for sample packages or short production runs. Credit: Rondo-Pak
A closer look at vials
Glass vials with a crimped aluminum cap are the standard for delivering injectable drugs, or at least those that have not been lyophilized (freeze-dried, which then necessitates a reconstitution step before administration) or supplied as prefilled syringes. Frequently, they represent the most expensive drugs available, containing biologic substances that must be carefully handled.
Much attention has been paid in recent years to prefilled syringes, which typically can be used by patients themselves and therefore represent a savings to healthcare systems and, for some patients, a desirable degree of self-reliance. Visiongain, a UK market research firm, published a recent study estimating the 2020 market size for prefilled syringe packaging at just under $7 billion in 2020, and predicts “strong growth” through 2026.
Even so, conventional vial packaging has seen its fair share of continuing development. Rondo-Pak (Camden, NJ) announced a “Quick-to-Market” carton assembly service at this year’s Interphex meeting (New York, NY, April 26—28) to enable manufacturers to provide sample packages, short or extended production runs, or backup packaging services with a quick turnaround. “Our Quick-to-Market carton assembly services are a reassuring security blanket for customers who find themselves challenged to fulfill a sudden need or unforeseen opportunity,” said Victor Dixon, president of Rondo-Pak, in a statement.
Meanwhile, there was considerable buzz in the business press about a study issued from Memorial Sloan Kettering earlier this year, highlighting the potential waste of expensive biologics that are provided in a limited number of vial sizes, even when those biologics are carefully calibrated for dosage by patient weight and other considerations. The study, “Overspending driven by oversized single dose vials of cancer drugs,” published in the British Medical Journal* made the point that somewhat arbitrary choices by manufacturers in the vial sizes can cause wastage of those drugs, since by usual pharmacy standards leftover medicine cannot be saved indefinitely for later administration. By some rough calculations of drug use, patient profiles and Medicare reimbursement data, the authors concluded that some $1.8 billion of 20 key injectable cancer drugs will be wasted this year. “Regulators could require manufacturers to provide drugs in a reasonable set of size options to ensure the amount of wasted drug is low, say 3%,” suggest the authors, or to “leave manufacturers free to select their vial sizes but also require them to refund the cost of leftover drug.”
This study presupposes that there is minimal or no additional cost to supplying and then distributing certain drugs in multiple vial sizes, let alone the complexity of stocking them at hospitals and pharmacies; and it was also a little vague on the practices of specialty pharmacies, where patient-specific dosages of drugs are usually compounded. But, in an era where closer and closer attention is being paid to both drug costs and pharmacy practices, it highlights the importance of better packaging decisions by manufacturers.
