Sensitech upgrades its real-time cold-chain tracking capabilities
SensiWatch Platform includes analytics and APIs for integrating supply-chain information
The SensiWatch Platform employs IoT data-collection instruments and data-management tools, the better to provide an end-to-end visibility of shipments, including both geo- and condition (temperature, humidity and light) tracking. Internally, SensiWatch uses cloud-based storage (building on its earlier ColdStream data-tracking services) a data warehouse, Application Programming Interfaces (APIs) for integrating with existing customer applications and third-party data sources, multiple levels of reporting and real-time analytics.
According to the company, basic reporting includes Supplier Network Overview, Segment Performance, Supplier and Carrier Compliance. Advanced reporting includes: Estimated Time to Arrival, Dwell Times, Simulated Product Temperature, Lane Risk Analytics, Weather Impact, and Crime Risk. “We’re now talking about good cold chain management, quality risk management, security, transparency, and authenticated chain of custody,” said Henry Ames, GM–Life Sciences. “The temperature map of each load is still critical, but we’ve taken a much more holistic view of supply chain integrity.”
There is an increasingly crowded arena for providing condition and location monitoring for pharma shipments. Historically, unconnected devices would be manually read out at the end of a shipment; now, there are monitoring services providing near real-time tracking from the freight forwarders themselves, from packaging or container providers, from instrument manufacturers like Sensitech, and from third-party telecom firms that seek to connect the devices, the carriers and the intermediary service providers. More competition means more choices for the pharma shipper.
The Ryan bimetallic 'thermostat', circa 1930, for cold-chain tracking.
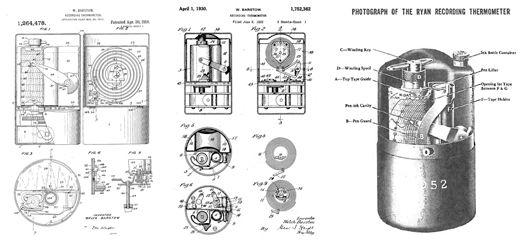
Simultaneously with this announcement, the company is taking note of its 30th anniversary and has posted a fascinating look backward on its evolution, as well as the general topic of condition tracking for temperature-sensitive materials. The technology can be traced back to Ryan Instrument Co. (which Sensitech acquired in 2000) and its strip-chart recorder for perishable food shipping. After its 1990 founding, Sensitech was acquired by United Technologies in 2006 and merged into a business unit that included Carrier Corp. In April, Carrier was spun out from UTC.