Secop Introduces ULT Cooling Compressor Concept
The finished product, company says, can assist with the medical supply chain’s vaccine distribution process.
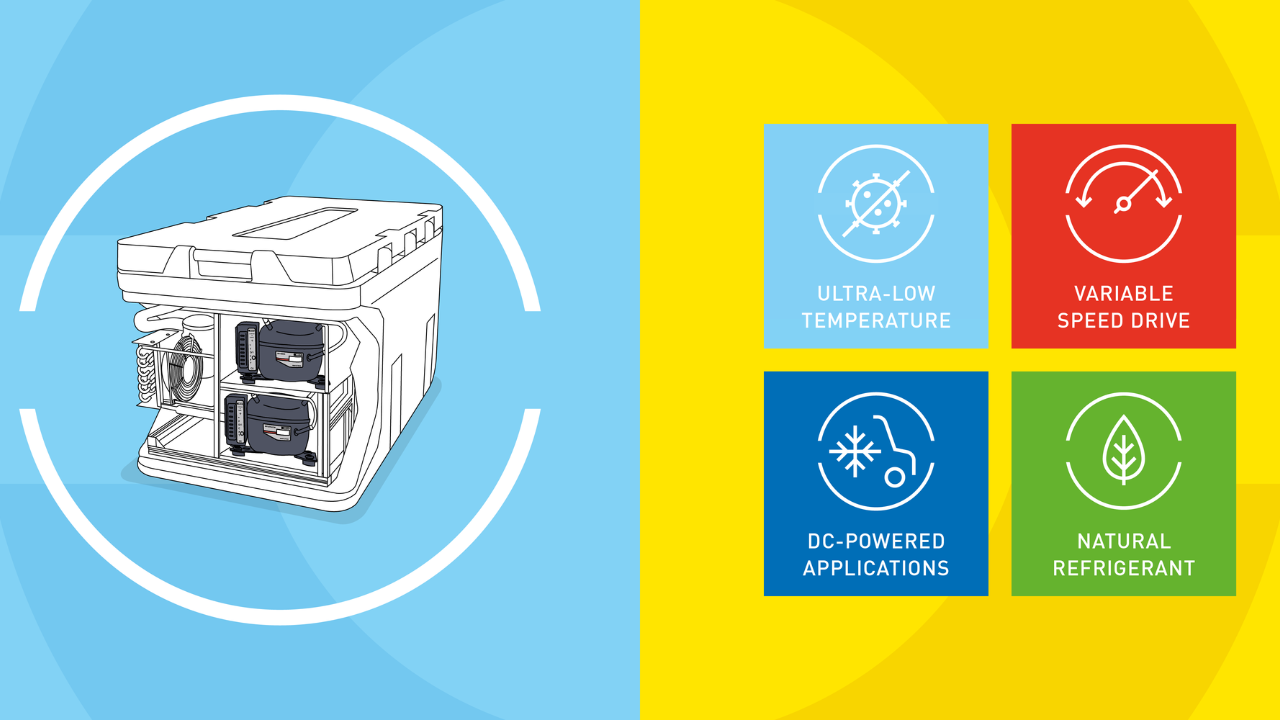
In order to streamline the storage and distribution of mRNA vaccines, Secop, who works in the medical refrigeration sector, is in the process of developing cooling compressors.
According to Secop, new mRNA vaccines require continuous refrigeration temperatures as low as -80°C, which presents a challenge to the transportation and distribution process. The journey of these vaccines begins with air transport from the manufacturer to various countries. Upon arrival, they are stored in specialized cold rooms before being distributed to regional cold storage facilities by refrigerated vehicles. From there, the vaccines are transported in coolers and vaccine carriers to various places, some of which include remote villages.
Keeping that in mind, the company has taken significant steps toward environmental sustainability by adopting refrigerants R290 and R170, which possess extremely low global warming potential (GWP) values. When it comes to storage applications, Secop offers a cascade configuration featuring either 2x MN13UVULTM or 2x MS18UVULTM medical grade variable speed AC compressors.