Sanofi, AstraZeneca Launch Program to Mitigate Future RSV Vaccine Shortages
New reservation program for Beyfortus (nirsevimab-alip) seeks to improve access to the monoclonal antibody that is FDA-approved to protect against respiratory syncytial virus-associated lower respiratory tract disease.
Image credit: Peter Hansen | stock.adobe.com
Sanofi and manufacturing partner AstraZeneca have announced a new reservation program to address the shortages of the respiratory syncytial virus (RSV) vaccine Beyfortus (nirsevimab-alip) that plagued the 2023–2024 RSV season.1 Beyfortus is a monoclonal antibody that protects against RSV-associated lower respiratory tract disease in newborns and infants under 8 months of age born during or entering their first RSV season.2
The Beyfortus Reservation Program seeks to improve readiness for the 2024-2025 RSV season with prioritized fulfillment of requests placed through the program, according to Sanofi.
“We are focusing on how we partner with customers with the aim of protecting babies from the leading cause of infant hospitalization in the US,” said Ayanna Santos, PharmD, Sanofi head of RSV Franchise, US Vaccines, in a press release. “We’re excited by the potential of the Beyfortus Reservation Program to help support planning for the next RSV season.”1
In October 2023, Sanofi announced that an unprecedented demand for Beyfortus led to a limited supply during the 2023–2024 RSV season, which prompted the CDC to issue a set of recommendations to address the shortages.2,3
“Despite an aggressive supply plan built to outperform past pediatric vaccine launches, demand for this product, especially for the 100 mg doses used primarily for babies born before the RSV season, has been higher than anticipated,” Sanofi said in a news release.2
Beyfortus was approved by the FDA in July 2023 for neonates and infants born during or entering their first RSV season, and for children up to 2 years of age with vulnerability to severe RSV disease through their second RSV season.4
The following month, the CDC Advisory Committee on Immunization Practices voted 10 to 0 to recommend the routine use of Beyfortus for the approved indications and voted unanimously to recommend its routine use in infants aged 8 to 19 months with an increased risk of severe RSV entering their second season.5
Demand for the vaccine prompted the CDC to recommend prioritizing available 100 mg doses of Beyfortus for infants under 6 months of age and for those with underlying conditions that increase their risk of severe RSV disease. The CDC did not alter recommendations for the 50 mg doses.3
In November 2023, the CDC and FDA announced the release of more than 77,000 additional doses of Beyfortus in an effort to increase availability of the vaccine to protect eligible children, prioritizing those with the greatest risk of severe illness.6
“Helping to ensure the availability of this preventative option to reduce the impact of RSV disease on eligible babies and young children, families and the health care system remains a priority,” Patrizia Cavazzoni, MD, director of the FDA’s Center for Drug Evaluation and Research, said in a press release. “We will continue to use all our regulatory tools to help bring safe, effective and high-quality medicines to the public.”6
Premature infants, specifically those with chronic lung disease related to premature birth or congenital heart disease, have the greatest risk for severe RSV. Approximately 1% to 3% of children under 12 months of age are hospitalized each year due to RSV, according to data from the American Academy of Pediatrics.4
2022-2023 respiratory syncytial virus cumulative hospitalization rates. Credit: USA Facts
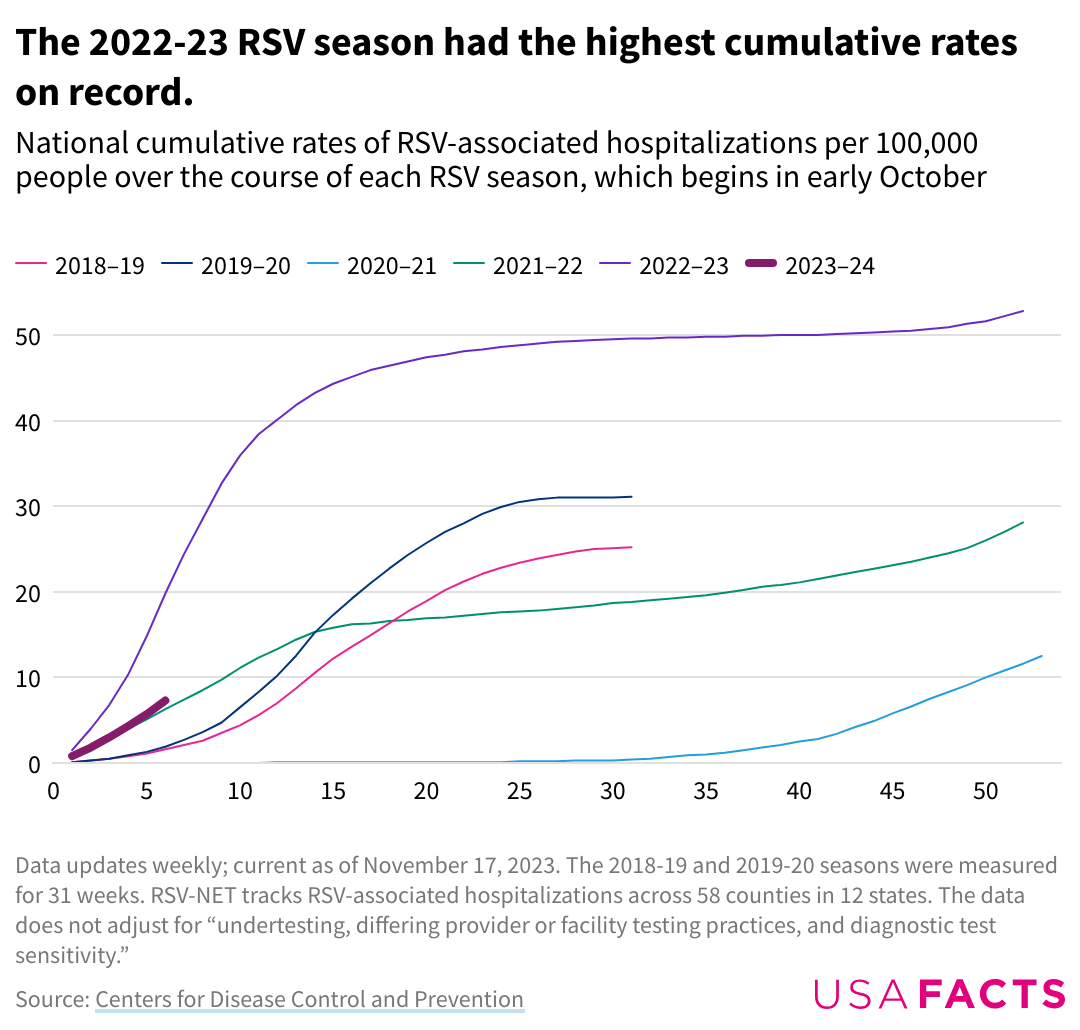
From the week of September 2, 2023, to the week of October 28, 2023, weekly RSV hospitalizations increased from 0.2 per 100,000 individuals to 1.3 per 100,000 individuals. The 2022–23 RSV season produced the highest cumulative hospitalization rates on record at 52.8 individuals per 100,000 over the 39-week season compared to 28.1 per 100,000 recorded in the 2021–22 season.7
In its announcement of the new Beyfortus Reservation Program, Sanofi stated that insurance coverage is in place for approximately 100% of US infant lives. The company said it will collaborate with private market healthcare providers and the Vaccines for Children program to ensure ongoing access to Beyfortus for eligible babies. The order window for those who order outside of the program will be available from September 2024 through February 2025.
“Beyond the Beyfortus Reservation Program for the private market, Sanofi is also taking additional measures to help ensure greater preparedness for the 2024-2025 season,” the company stated in a news release. “Sanofi will continue to work with AstraZeneca to expand the Beyfortus manufacturing network to allow for a significant increase in supply for the 2024-2025 season and cover the entire US. demand. Beyfortus is being manufactured well in advance of the RSV season, with the vast majority of doses expected to be available before October.”1
References
1. Sanofi launches Beyfortus® (nirsevimab-alip) Reservation Program for 2024-2025 RSV season in U.S. Sanofi. News release. February 1, 2024. Accessed February 2, 2024. https://www.news.sanofi.us/2023-02-01-Sanofi-launches-Beyfortus-R-nirsevimab-alip-Reservation-Program-for-2024-2025-RSV-season-in-U-S
2. Limited Availability of Nirsevimab in the United States—Interim CDC Recommendations to Protect Infants from Respiratory Syncytial Virus (RSV) during the 2023–2024 Respiratory Virus Season. US Centers for Disease Control and Prevention. News release. October 24, 2023. Accessed February 2, 2024. https://emergency.cdc.gov/han/2023/han00499.asp
3. Sanofi Beyfortus Statement. Sanofi. News release. October 24, 2023. Accessed February 2, 2024. https://www.news.sanofi.us/Sanofi-Beyfortus-Statement
4. FDA Approves New Drug to Prevent RSV in Babies and Toddlers. News release. FDA. July 17, 2023. Accessed February 2, 2024. https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-prevent-rsv-babies-and-toddlers
5. U.S. CDC Advisory Committee unanimously recommends routine use of Beyfortus™ (nirsevimab-alip) to protect infants against RSV disease. Sanofi. News release. August 3, 2023. Accessed February 2, 2024. https://www.news.sanofi.us/2023-08-03-U-S-CDC-Advisory-Committee-unanimously-recommends-routine-use-of-Beyfortus-TM-nirsevimab-alip-to-protect-infants-against-RSV-disease
6. CDC and FDA Expedite the Availability of Additional Doses of New RSV Immunization for Infants. News release. Centers for Disease Control and Prevention. November 16, 2023. Accessed February 2, 2024. https://www.cdc.gov/media/releases/2023/p1116-rsv-doses.html
7. What is the state of RSV in the United States? USA Facts. Website. December 5, 2023. Accessed February 2, 2024. https://usafacts.org/articles/what-is-the-state-of-rsv-in-the-united-states/