Innovative models to address cell- and gene-therapy (CGT) payer challenges
Carving out CGTs for payment systems involving government intervention may be an answer
In the past two years, four new cell and gene therapies have entered the US market at unprecedented prices. Most recently, Zolgensma launched at a price of $2.1 million per treatment. Payers are concerned about the high cost, especially since it is borne all at once. In addition, they express uncertainty about the long-term durability of effect of cell and gene therapies.
To date, there have been limited requests for CGTs. However, as utilization expands to broader patient populations and as more CGTs are approved across additional indications, the budget impact will grow. As a result, new and innovative payment approaches may need to emerge to pay for these high cost, high value, “one and done” therapies.
To that end, Two Labs conducted in-depth qualitative telephone interviews with 14 US payers representing commercial, Medicare, and Medicaid lives. The research was completed in September 2019. During this survey, payers were asked an unaided, open-ended questions to suggest payment solutions for CGTs. Payers suggested various options including reinsurance, risk-based contracts, and value-based pricing.
Survey results were based on open-ended, unaided responses. credit: Two Labs
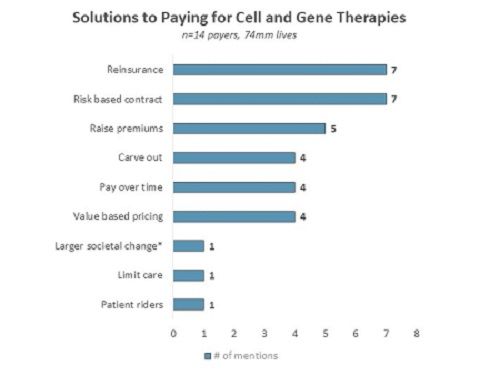
Reinsurance: Reinsurance, a model where a larger entity above the insurance company would provide stop-loss insurance to handle high-cost CGT claims, is seen as a feasible solution. Payers note that there is already a precedent for this model in other industries, for example in crops and flood. However, the biggest concern with this model is finding an entity large enough to take on the risk. This could require government intervention. For example, a Medical Director at a large national plan notes: “There are standard ways that insurance companies handle these difficult and unpredictable one-time events and risks. It is called reinsurance or insurer of last resort. I do not think there is any single entity that is big enough to take on the financial risk of gene therapy. It would have to be the government.” In addition to suggesting reinsurance as a possible payment solution, payers suggest the idea of patient-rider and carve-outs which are similar ideas in the sense that they are all ways of separating out cell and gene therapies from other therapies.
Risk-based contracts: Two risk-based contracts explored in this research were a one-year performance-based contract and a five-year annuity payment based on performance at the end of each year. Payers express concern that the one-year model does not provide a long enough time horizon to truly understand long-term efficacy. A five-year model is considered more robust, but due to patients switching insurance every few years, it would be difficult to track patient outcomes over a five-year time horizon.
Value-based pricing: Payers echo a common theme that while innovative payment solutions sound interesting, their preferred solution would be for pharmaceutical manufacturers to price in line with expected long-term outcomes. In the US, the Institute for Clinical and Economic Review (ICER) conducts cost-effectiveness analysis on newly launched drugs. ICER reviews are beginning to play a larger role in payer discussions, but they are ultimately not a decision-making factor.
The approval of CGTs is expected to accelerate over the next 5-10 years. Finding a solution that fits the needs of payers, manufacturers and patients will require cooperation between all parties, and possibly even government intervention. Eventually, the system will catch up and optimal payment solutions will be put in place.
About the author
Anne Runyan is Senior Consultant, Market Access, at Two Labs Pharma Services