Drug Shortages: A Chronic Problem, but New Opportunities Present Themselves
While this concern is causing ripples in the global supply chain, technological and regulatory advances can help address the issue
Medicines are a vital component of human well-being, and access to medicines has increasingly become a fundamental part of daily life. Yet, shortages of pharmaceutical medicines have been a chronic issue facing the pharmaceutical and healthcare industry for well over a decade. It may seem surprising that even in the US, with its advanced pharma supply chain, drug shortages are an issue. But, as is well documented by the FDA, the American Society of Health System Pharmacists (ASHP), and other organizations, since 2008, there has been an average of over 140 drug shortages reported annually (see graph below).
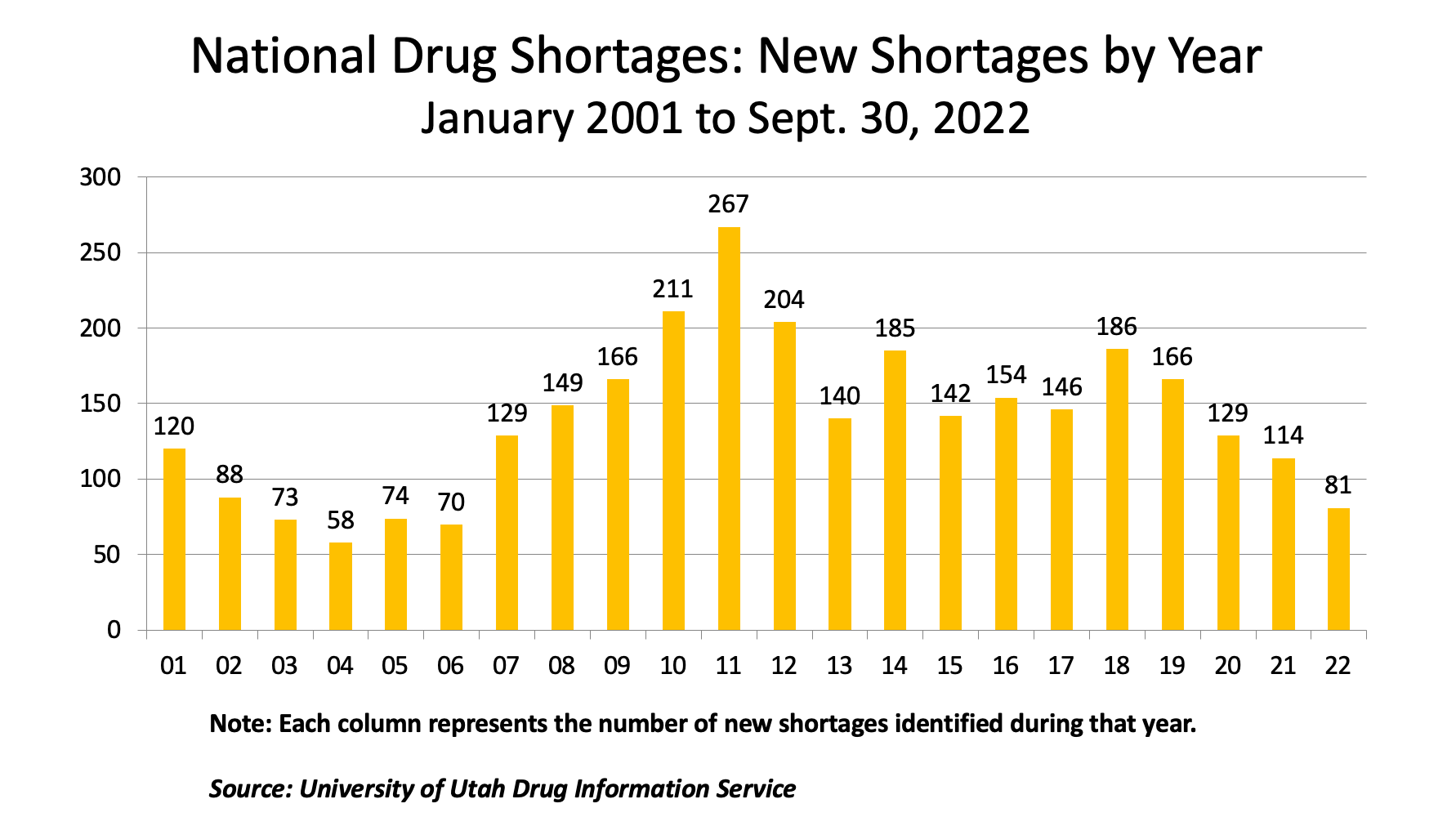
A shortage of medicines creates myriad issues for humans and companies alike. For patients, medical procedures may be delayed or canceled, treatment outcomes may be impacted due to the need to temporarily or permanently switch medications, and hospitalization stays may be lengthened. For hospitals and pharmacies, shortages drive up costs as alternative medicine regimens need to be created and alternative drugs need to be sourced. Organizations have in many cases seen a 15-30% increase in drug sourcing costs for generic alternatives.
In addition, patient trust in the healthcare system is diminished. Pharma companies are impacted, as initial sales are lost for a drug in shortage, while long-term revenue is lost when substitute medicines become permanently switched in the healthcare system, and producers of alternative drugs face unexpected, yet potentially temporary, demand to fulfill. At every point in the supply network—from ingredient and raw material to the patient—the impacts are felt.
The critical and strategic nature of drug shortages became apparent at TraceLink back in 2020 through TraceLink’s Collective Intelligence Executive Forum, a collaborative group of senior executives spanning the entire supply chain from marketing authorization holders/biopharma companies, contract manufacturers, wholesale distributors, retail pharmacies, and healthcare organizations, the Collective Intelligence Executive Forum studied several pressing issues facing the pharma and healthcare supply chain during the global pandemic. Between 2020 and 2022, the Forum ranked the understanding and early warning of drug shortages as the top priority. Through further discussions with pharma manufacturers and healthcare systems on the TraceLink Network, it became clear that this was a diverse and pervasive problem.
Underlying issues and drivers constraining medicine availability
The overall causes of drug shortages are numerous, but can be generally grouped into supply issues, demand issues, regulatory issues, and combinations of these factors. Raw material or active pharmaceutical ingredient (API) access may be uncertain in the global supply chain.
Quality issues may arise during a manufacturing run or medicine shipment. Unanticipated demand may spike due to unforeseen medical issues, such as with COVID-19. Emerging regulations may impact medicine production or efficiency in the supply chain until organizations adjust their operations.
Perhaps more importantly, these underlying causes create major impacts on the participants of the supply chain. These include, but are not limited to:
- Lack of visibility by manufacturers into product consumption and potential supply chain signals, which may indicate emerging supply constraints or outright drug shortages
- Lack of early warning signals for pharmacies and hospitals into potential drug shortages, thus preventing them from taking proactive measures
- Lack of insight for pharmacies and hospitals into the intensity and duration of current drug shortages as reported by authorities such as the FDA and ASHP, thus limiting the scale, scope, and effectiveness of mitigating actions that are taken
- Fundamental lack of getting the right information to the right people at the right time at multiple points across the supply chain to positively impact an impending supply/demand imbalance
As we have seen, drug shortages are not new, nor are the underlying causes or their supply chain impacts. The question is, why now do we have an opportunity to tackle this critical issue?
Bharath Sundararaman

New opportunities emerging for the fight against drug shortages
The recent convergence of technological innovations, supply chain process changes, and regulatory pressures, particularly in the US, was one area that the Forum examined in determining that we have reached a tipping point in the fight against drug shortages. New data sources and technology adoption are emerging which can provide an effective, systematic approach towards directly mitigating drug shortages. In addition, these recent innovations and their incorporation into business-as-usual supply operations can provide a fundamental foundation toward improving the overall ability of the end-to-end supply chain in ensuring that patients get the medicines they need.
Part of this has been driven by the implementation of the US Drug Supply Chain Security Act (DSCSA), a law designed to help enhance pharma supply chain security in the US. DSCSA requires that pharma manufacturers, repackagers, distributors, and healthcare organizations/pharmacy dispensers implement new product identification/labeling, tracing, verification, and other regulations in their production, distribution and other supply chain operations. The phasing in from 2015 to 2023 of the product/transaction data creation and capture requirements, network integration needs, and electronic data exchange capabilities driven by DSCSA has created a unique opportunity in the US pharma supply chain and other geographies with similar requirements.
In parallel, several new innovations have arisen which now enable healthcare organizations, retail pharmacies, distribution partners, and pharmaceutical manufacturers in particular to take advantage of the investments driven by DSCSA, including:
- Supply chain digitalization, deployed on a collective platform, which significantly enhanced the network linkage of, and the data exchange between, hundreds of thousands of network participants, from manufacturer to pharmacy and hospital
- Development and deployment of a high performance, cloud-based network platform that can handle the mass scale of near real-time product, transaction, and participant information
- Collective intelligence fed by the creation and exchange across the network of interoperable transactional product data, including National Drug Codes (NDCs), which is readily available in traceability repositories and network systems
- Maturation of artificial intelligence (AI) and machine learning (ML) tools which can be applied to the newly available network data sets, driving deep insights which can be applied to supply decision-making
Collective, collaborative actions to test and innovate on product availability
TraceLink and its pioneering industry collaborators started early to design a technological approach and supporting program focused on the potential ability to tackle drug shortages. This involved the use of collective intelligence fed by a wide variety of publicly available and anonymized TraceLink network data sets to inform a Product Availability Intelligence (PAI) solution. The goal of this solution was to gather critical early insights, test drug shortage prediction models, and drive rapid iterative innovation to forecast when the next product shortage may occur.
The collective team laid out several specific near-term and longer-term benefits anticipated from the use of PAI solutions and related supply chain operational enhancements for all participating supply chain members. These included:
- Identification of medicines which may be at risk for being in short supply
- Developing a profile and timeline for the potential drug shortage, including its predicted start and duration
- Notification of potentially affected parties towards the emerging short supply condition
- Identification of mitigating supply actions which can be taken to eliminate altogether or lessen the impact of a drug shortage
- Identification of additional short-term mitigating actions which may be available, such as identification of alternate supply sources and alternate medicines
- Driving better forward-looking collaborative supply chain agility to reduce the future risk of drug shortages and supply issues
The initial question was a simple one: Can we predict drug shortages, and if so, under what conditions? To this end, after two years of development, the initial deployment of PAI began in Q2 2022, in a highly controlled environment, to test permissioned data, business rules, and learning models against existing historical data on current drug shortages. Based on early evidence developed through rigorous testing, the solution and platform continue to be refined. The rest of 2022 has been dedicated to a focus on forward prediction models that are now being tested to validate the ability of PAI to anticipate a future drug shortage. The goal of this phase is to increase the confidence in a drug shortage prediction, and to increase the forward lead-time of a prediction against the predicted onset of a shortage.
The early evidence is very promising on the emerging ability to anticipate and measure potential drug shortages, and to also take proactive, corrective actions to help prevent such shortages from occurring in the future. Yes, the light at the end of the drug shortage tunnel is certainly starting to come into clearer view, one step at a time.
About the Author
Bharath Sundararaman is General Manager, Intelligent Supply Network, TraceLink.