Aurlumyn Gets First FDA Approval for a Severe Frostbite Medication
Patients with severe frostbite who were administered Aurlumyn monotherapy experienced a significantly reduced risk of the need for amputation.
Image credit: Szymon Bartosz | stock.adobe.com
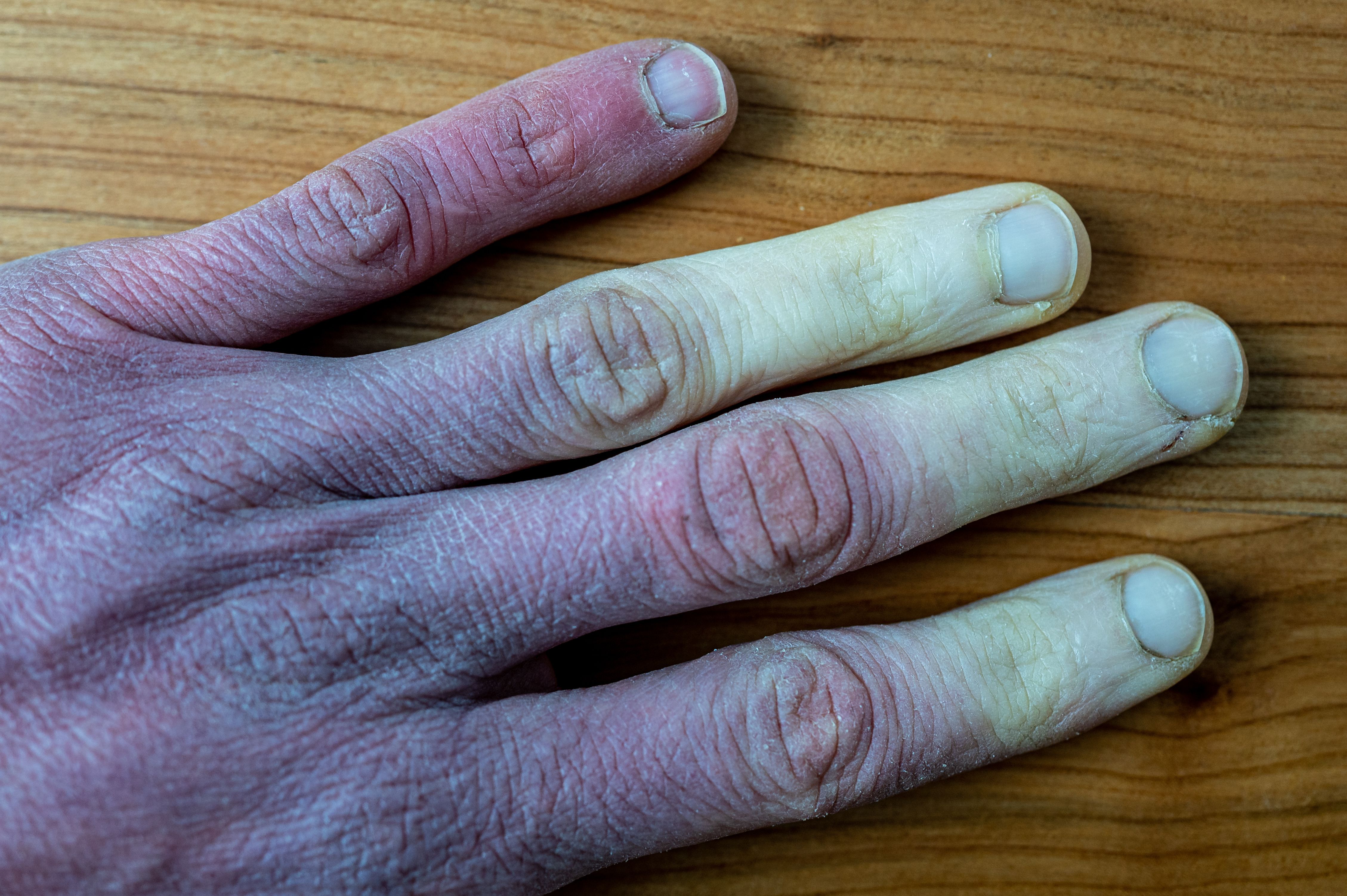
The FDA announced that it has officially approved Actelion Pharmaceuticals’ Aurlumyn (iloprost) injection, the first treatment cleared for severe frostbite in adults, with the intent of reducing the likelihood of amputation of fingers or toes. Aurlumyn, a vasodilator that prevents blood clotting, demonstrated efficacy in a controlled trial involving 47 adults with severe frostbite. The trial investigators found that patients administered Aurlumyn monotherapy experienced a significantly reduced risk of the need for amputation compared to those who received another form of treatment. Aurlumyn, the pricing of which has yet to be released, is expected to be available this upcoming spring.1,2
"This approval provides patients with the first-ever treatment option for severe frostbite," said Norman Stockbridge, MD, PhD, director, division of cardiology and nephrology, FDA's Center for Drug Evaluation and Research, in the aforementioned press release. "Having this new option provides physicians with a tool that will help prevent the lifechanging amputation of one's frostbitten fingers or toes."
Aurlumyn was previously granted FDA Priority Review and Orphan Drug designations for treatment of severe frostbite in adults. The FDA based its approval of Aurlumyn on findings from a controlled, open-label trial that enrolled 47 adults with severe frostbite.3
Those enrolled in the trial were randomly assigned into three groups—one of which was administered Aurlumyn monotherapy intravenously for six hours per day for up to eight days, while the other cohorts were administered other treatments that have not been approved to treat frostbite. These treatments were administered both with and without Aurlumyn. All patients enrolled in the trial also received aspirin intravenously and standard of care. The primary endpoint of the trial was a bone scan obtained seven days following the initial frostbite, which was used to predict the need for amputation of at least one finger or toe.3
After seven days, the bone scan that was predictive of the need for amputation was not observed in those administered Aurlumyn monotherapy. The other two cohorts had scans that predicted the need for amputation. The most common adverse events observed in individuals administered Aurlumyn were headache, flushing, heart palpitations and fast heart rate, nausea, vomiting, dizziness, and hypotension.3
Back in 2004, Actelion initially received FDA approval to treat pulmonary aerial hypertension (PAH), with development led by California company CoTherix. The product at that time was known as Ventavis and it was the only inhaled therapy for PAH available in the United States.2 Despite the approval, Aurlumyn reportedly has the potential to cause symptomatic hypotension.4
Frostbite can occur at any temperature below freezing. The probability of suffering from frostbite depends on factors such as age, clothes being worn, and drug intake. According to Very Well Health, babies, children, and older adults have a higher risk for frostbite than younger adults. Additionally, health conditions that lead to poor circulation include diabetes and atherosclerosis.5
Signs of frostbite often include as follows:
- Numbness
- Skin changes colors
- Swelling
- Frozen skin.5
Currently, healthcare providers will attempt to treat frostbite by rewarming the areas affected with warm water. In some cases, surgery will be required in order to relieve pressure associated with compartment syndrome.5
References
- FDA Approves First Medication to Treat Severe Frostbite. PR Newswire. February 14, 2024. Accessed February 14, 2024. https://www.prnewswire.com/news-releases/fda-approves-first-medication-to-treat-severe-frostbite-302061881.html
- FDA Approves First Treatment for Severe Frostbite. Newsmax. February 14, 2024. Accessed February 14, 2024. https://www.newsmax.com/health/health-news/frostbite-severe-treatment/2024/02/14/id/1153595/
- FDA Approves First Medication to Treat Severe Frostbite. FDA. News release. February 14, 2024. Accessed February 14, 2024. https://www.fda.gov/news-events/press-announcements/fda-approves-first-medication-treat-severe-frostbite#:~:text=Today%2C%20the%20U.S.%20Food%20and,of%20finger%20or%20toe%20amputation.
- UPDATED: Eicos Sciences' Aurlumyn scores first FDA approval to treat severe frostbite. Fierce Pharma. February 14, 2024. Accessed February 14, 2024. https://www.fiercepharma.com/pharma/johnson-johnson-scores-first-fda-approval-treat-severe-frostbite
- Frostbite: Treatment for Mild to 1st-Degree Symptoms. Very Well Health. December 19, 2023. Accessed February 14, 2023. https://www.verywellhealth.com/frostbite-8402471