Another go at value-based therapy models for CGTs
ARM-sponsored study projects a 23% reduction in the cost of treating blood diseases in 2029 by administering cellular and genetic therapies
The cellular and genetic therapies introduced so far are expensive, with some ranging over $2 million. The saving grace, however, is that most of these treatments, for at least some patients, are curative: the disease is essentially eliminated. How to reconcile the high up-front cost with the longterm benefit, given that someone (the insurer) must make the payment, has been the dilemma that policymakers have been dealing with.
A new study, sponsored by the Alliance for Regenerative Medicine and performed by Marwood Group, a New York healthcare advisory firm, tackles the dilemma with two new elements; analyzing a projected cohort of patients over the 2020-2029 period for three key CGT therapies; and factoring in the lost productivity for both patients and their caregivers when diseases are treated only with conventional therapies. The three therapies are for multiple myeloma, sickle cell disease, and hemophilia A. Marwood estimates there are 172,000 patients in the 2020-2029 cohort (mostly in myeloma). It also estimates, based on current CGT costs, a “price corridor” of $1.57 million for sickle cell or hemophilia CGT therapy, and $373,000 for myeloma. (Marwood tested various price points in its modeling, and ARM emphasizes that it “does not intend to recommend price points to industry or governments.”)
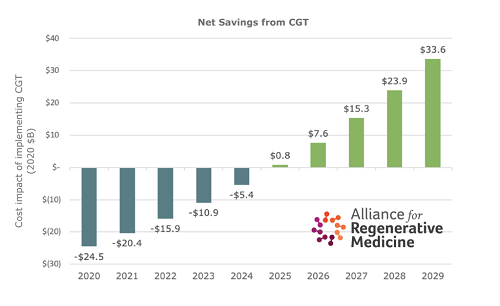
When the annual costs of treatment, conventional vs. CGT, are marched out over the decade (including such factors as less-than total cure rates, medical cost inflation and others), Marwood finds that there is a break-even at year 5, and a net cost savings for CGTs thereafter, reaching as much as $33.6 billion in year 10 (2029). (See figure.) Some $3.5 billion of that comes from improved productivity of formerly sick patients, and their caregivers, who could now work.
Marwood and ARM contrast this model with those based on QALYS (Quality Adjusted Life Years), which, generally speaking, do not factor productivity gains into the evaluation. The Marwood model also lends itself to payer approaches such as an annual subscription cost, versus the one-time up-front cost, as a way to reduce sticker shock and to identify cumulative savings to healthcare. According to Marwood, a price sensitivity analysis of the model demonstrated that the cost of therapy is outweighed by access to therapy; in other words, the price of the drug was less significant than the volume of patients who might have access to the therapies.
“It is imperative that payers, both government and commercial, and other stakeholders adopt new value-based paradigms that reflect the benefits of a durable or curative therapy in place of a palliative care approach that simply treats the symptoms, rather than the root cause of disease,” says Janet Lambert, CEO of ARM. “The model used in this report provides an innovative framework for evaluating the potential direct and indirect cost-savings these therapies offer in comparison to the current standard of care.”











